Document Type : Review Article
Authors
Department of Chemistry, University of Wah, Quaid Avenue, Wah Cantt., (47010), Punjab, Pakistan
Abstract
Fruits include nutrients that are essential for good health as well as body repair. Heavy metals occur spontaneously in the environment as a result of natural sources and anthropogenic activities or contamination through industrial operations, preservation, including cooking. Pesticide pollution is a worldwide environmental and public health hazard since pesticides are carcinogenic and extremely poisonous among all living species, especially plants, animals, even people. Pakistan, an agricultural-based country, uses a large quantity of such organochlorine-pesticides (OC) each year to manage insect pests and various crop diseases, which is health risk. However, contemporary statistics on pesticide usage, pollution, and exposure in Pakistan are mainly missing. This study highlights recent findings and gives statistics on pesticide usage and pollution, with a special emphasis on human exposure. Mango considered one of Pakistan's most important fruit crops, yet it is facing threat from the World Trade Organization (WTO). To that end, the current study was carried out to examine agricultural extension programs in mango marketing and production with specific reference to the WTO. It was discovered that respondents' knowledge of the different WTO agreements was inadequate, and in certain cases non-existent. According to respondents, the positive influence of WTO would be beneficial in providing refined products at the customer’s doorstep and reliable to human and environmental life, but the primary threat of cumulative authority over marketplaces by developed nations through their better technology and very well-equipped industry, related specifically to mango fruit and its byproducts.
Graphical Abstract
Keywords
Main Subjects
- Introduction
In addition to crop output, costs, and yields, agricultural productivity heavily depends on how quickly a commodity leaves the farm and makes its way to the consumer. Each of these tactics helps to boost agricultural output while also encouraging more people to eat the foods produced. One of the more well-liked and widely consumed fruits in Pakistan is the mango, scientifically known as Mangifera indica. It tastes delicious and has a distinct aroma. Due to its distinctive qualities, it is referred to as both the "Honeydew of God" and the "King among Fruits." In addition to other nutrients like protein, sugar, crabs, and organic acids, it is high in vitamins A and C, and other minerals. Mango was initially mentioned to be grown in Eastern India, Pakistan, Burma, and the Andaman Islands [1]. It flourishes best around 250 N as 250 S of equator, which indicates that primary mango growing locations are Pakistan, Burma, and India.
Pakistan ranks the fifth around the world for mango output, despite having the finest variety of agricultural production parameters for fruit cultivation, whilst India and mainland China boosted their production over the last decade. Mango is mostly grown in the Punjab regions of Multan, D.G Khan, Bahawalpur, Lahore, Faisalabad, and Sargodha. Mangoes come in over 100 different types, each with its own distinct taste, flavor, color, tinges, form, and size. Famous types include Chaunsa (Sammar Bahisht), Dusehri, Langra, Sindhri, Anwar Ratole, and others. Their worth and excellence have already been evaluated and acknowledged at the local and international level. It has seasonal range, with some types being early, mid, and late.
Mango has a potential output per acre of 21.6 metric tons, which is greater than the typical yield of 9.96 metric tons [2]. This demonstrates a significant difference, which could be credited to various factors such as mango arthropod such as mealy bug and sometimes fruit fly, diseases such as powdery mildew, poison hemlock, and die back; not only weather hazards, a deficient marking system, but also poor post- and pre-extract practices. Extension services for agriculture are primarily responsible for knowledge distribution and adaptation. The World Commerce Organization's Agriculture Agreement seems to be the primary international agreement that regulates global trade in food goods. The Agreement covers three major areas:
- Better market access,
- Reduction of domestic farming assistance, and
- Decline of export subsidy.
Agriculture-related agreements, such as customs valuation, compared to pre-shipment inspection, technically trade barriers, import licensing, sanitary as well as phyto-sanitary countermeasures, safeguards, subsidies and compensatory measures, tariffs, anti-dumping legislation, and so on.
All preceding materials suggest that the WTO adoption in the next years will exacerbate the problem. WTO stressed the need of implementing its suggestions such as sanitary as well as phyto-sanitary requirements, the prohibition of toxic chemicals, post in addition to pre-harvest procedures, export, and including quarantine legislation. This deplorable condition, particularly in relation to mango, will worsen, and mango producers may confront profit return issues. As a result, it is required to perform a study to investigate the obstacles and challenges encountered by researchers, development service providers, marketing professionals, and mango producers as an outcome of the implementation of Treaty provisions [3].
A nutritious and healthful diet must include fruits and vegetables, but the health advantages are diminished by persistent pesticide contamination [4]. Pesticides are chemicals used in agricultural, home, and institutional settings to eradicate animal, insect, plant, and fungal pests [5]. Due to mounting evidence of the carcinogenic, mutagenic, and teratogenic consequences of pesticides in exposed humans and experimental animals, interest in pesticide toxicity has grown significantly over the past few years [6]. Due to their extreme toxicity and widespread usage in agricultural operations for field and post-harvest protection, they make up a significant group of chemical compounds that should be managed [7]. Pesticides are mostly introduced into the general population through the consumption of contaminated foods (such as cereals, vegetables, and fruits), which are either directly treated with these pesticides or cultivated in polluted fields. In aggregate and cumulative risk models, diet is one potentially substantial source of pesticide exposure [8]. Organophosphate, organochlorine, and related insecticides work by binding to the enzyme acetyl cholinesterase, causing nerve activity to be disrupted, resulting in paralysis and sometimes death [9]. They can cause both acute and chronic poisoning. Miosis, urine, diarrhoea, diaphoresis, lacrimation, CNS stimulation, and salivation are the acute consequences. Chronic exposure has neurological and behavioral consequences. Pesticides can cause cancer, allergies, hypersensitivities, central and peripheral nervous system damage, reproductive abnormalities, and immune system disturbance [10]. Recent research indicates that pollutant exposure in food may constitute a public health concern [11]. Children may be more vulnerable to the impacts of these exposures than adults because they have faster metabolic rates, less mature immune systems, and different patterns of activity and behavior [12].
Pesticides can potentially disrupt medication metabolizing enzymes, particularly Cytochrome P450, resulting in drug interactions [13]. Pesticides applied on vegetable and fruit crops account for around 27% of pesticides eaten in Pakistan. It is especially vital for emerging nations to strike a balance between risk and strategies for increasing agricultural output [14]. Pesticide residue monitoring in food is currently a primary goal in pesticide research to provide a comprehensive evaluation of food quality and avoid any dangers to human health. To guarantee the safety of the US food supply, the Environmental Protection Agency (EPA) establishes a tolerance or maximum residue limit (MRL), which is the amount of pesticide residue that may legally remain in each pesticide-treated food product [15]. The maximum residue limits (MRLs) for pesticides in a range of foods have been determined at the international level by the Codex Alimentarius Commission of the United Nations Food and Agriculture organization and the World Health Organization (WHO) [16].
- Diseases and Toxicity in Mango Fruits
Following are the disease considered in mango (Mangifera indica), as depicted in Figure 1.

Figure 1. Diseases and toxicity due to different pests in Mango
2.1. Mango mealy bug
Due to its polyphagous habit, the mango mealy bug, Drosicha mangiferae, is one of the most problematic insect pests affecting mango in Pakistan. It lays eggs in loose soil within a 2-3 meter radius of afflicted trees. When the temperature rises, the eggs hatch, and the nymphs crawl to the succulent shoots and fruiting sections. Nymphs and female bugs feed on the sap of the inflorescence, sensitive leaves, shoots, and fruit peduncles. As a result, the damaged inflorescences wilt and dry out. Infestation is severe, affecting fruit set, and causing fruit drop. They secrete honey dew on the leaves, which causes sooty mould to [17].
One of Pakistan's most significant fruit crops and a major source of foreign cash is the mango, Mangifera indica L, a member of the Anacardiaceae family. The great variety of agro-climatic conditions that nature has given Pakistan allow for the high-quality cultivation of both tropical and temperate fruits. All fruit varieties thrive in Pakistan's climate. Pakistan's second-largest fruit crop, after citrus, is mango. Pakistan is now ranked fifth in the world for mango output overall [18]. In the current year (2010), production has significantly dropped to 9-10 tons per acre. It amounts to around half of the 20 tons per acre theoretical production, which demonstrates a considerable disparity in average and prospective yields. The major cause of the productivity decrease is insect and disease impact on mango plantations.
Various insect pests are known to affect mango plants that have been well examined. Several of them are undoubtedly responsible for significant damage and have become a limiting issue in many mango-growing locations. Growers should initially be informed of the sorts of insect pests they are likely to face before conducting frequent surveys to successfully monitor a mango orchard for insect pest outbreaks [19].
2.1.1. Toxicity due to insecticide against mealy bug
Spinosad (Tracer®, 240SC; Dow AgroSciences, Hitchin, UK), imidacloprid (Confidor®, 20SC; Bayer Crop Sciences, St. Pierre Lyon, France), emamectin benzoate, and chlorpyrifos (Lorsban® 40EC; Dow AgroSciences) were pesticides contrasted with deltamethrin and bifenthrin (Talstar, 100 gram active component per liters EC; FMC, Philadelphia, PA, USA). That kind of pesticides' comparative efficacies and lethal times (LT) alongside all of H. mangiferae-evaluate. These insecticides were taken into consideration for various mechanisms of action in addition to the potential to use them in a strategy to control H. mangiferae's tolerance to pyrethroids [20].
2.2. Hypocryphalus mangiferae Stebbing
Infected mangos with Mango Sudden Death Syndrome, or MSDS, were found to have Hypocryphalus fact of nature Stebbing (Coleoptera: Scolytidae) the most often in the beginning phases, while Xyleborus sp. appeared later but in less numbers [21]. Several bark beetle genera are considered to be implicated as possible vectors of MSDS, which is acknowledged as one of the most serious issues not just in Pakistan, but across the world's mango-growing countries [21-23]. Xyleborus sp. is really insect vector observed in evergreen fruit trees with Ceratocystis canker brought upon with Ceratocysis fimbiriata that also wants to invade the xylem parenchyma below inner bark (phloem). In "vascular wilts" like Dutch elm disease, that was believed to be spread by countless elm insect pests of species Scolytus throughout Europe, North America, and Central Asia.
A number of biological traits of bark beetles, together with monitoring and control techniques, have been investigated as potential threats to the MSDS dissemination. On the other hand, there have not been many investigations into the genetic diversity of mango bark beetles, which might advance molecular ecology investigation and eventually lead to the creation of even more potent management strategies.
A new species hypocryphalus is called hypocryphalus mangiferae. Stebbing, among the most destructive predatory insects of mango plants, has been connected to the spread of the organisms that cause the illness in the plants to die suddenly. The goal of this study was to evaluate the cytotoxicity of spinosad, emamectin benzoate, imidacloprid, bifenthrin, deltamethrin, and bifenthrin in laboratory and field trials [24].
2.3. Insecticide-related toxicity against Hypocryphalus mangiferae
Emamectin benzoate (Syngenta Crop Protection, Greensboro, North Carolina, USA), imidacloprid (Bayer Crop Sciences, St. Pierre Lyon, France), Spinosad (Dow Agro-Sciences, Hitchin, UK), and chlorpyrifos (Dow Agro-Science) pesticides were compared to the widely used insecticides bifenthrin (100 g active component L-1, Philadelphia PA, USA) and permethrin (Bayer Crop Sciences). We evaluated the relative efficacies of these insecticides against H. Mangiferae and the standard deviation of lethal time (LT) values. Based on their mechanisms of action and potential for use against H. Mangiferae in a resistance management plan should the pest develop Pyrethroid resistance, insecticides were chosen.
In laboratory and field tests, the dangers of cypermethrin, imidacloprid, deltamethrin, chlorpyrifos, spinosade, and mamectin benzoate were evaluated. According to bioassay data, the acute toxicity of chlorpyrifos appears to be significantly higher than those of deltamethrin but similar to those of bifenthrin. On the contrary, deltamethrin, and bifenthrin's toxicity increased noticeably (P<0.01) from day 1 to day 3. The least poisonous substance found was spinosad, whereas the most toxic substance, emamectin, showed a sharp increase in toxicity from day 1 to day 5. When the therapeutic efficacy of the insecticides were evacuated using lethal durations to create 50% mortality (LT50) and spinosad, chlorpyrifos, imidacloprid, emamectin, and spinosad, their comparative potencies were higher than those of bifenthrin and deltamethrin (LT90). The results of something like the field testing showed that 12% of the bifenthrin-treated twigs had significantly fewer insects than the control twigs (P<0.05), which had the highest initial beetle appearance rate. The results of the recent study suggest that surface insecticides and chlorpyrifos may be used as an alternative to deltamethrin and bifenthrin as part of a comprehensive H. mangiferae action plan to eradicate the beetles off mango plantations.
2.4. Bark and ambrosia beetles (Scolytidae: Coleoptera)
Bark and ambrosia beetles are two of forest insects with the greatest economic importance (Scolytidae:Coleoptera). Worldwide, tropical in addition to subtropical regions have been home to over 6000 different scolytid species [25]. Beetles frequently reproduce on woody plants and leave behind recognizable tunnels inside the wood or pith (bark beetles or ambrosia beetles) [24]. The majority of the time, bark beetles act as a caustic agent to aid in the penetration of all mango fungus into the vasculature, which causes pathogen infection and transmission to the mango tree [22]. Nuclear microsatellites might well be transferred between species, enabling correlations between similar species for investigating the process of diversification, diverging, and diversity just at genetic and community levels, according to research on the bark beetle [26-28]. As a consequence, we looked at a panel of such microsatellites created in several scolytid microorganisms for cross amplification on H. mangiferae and Xyleborus affinis which were being connected with ill mango plants. Populations of mango bark beetles, Hypocryphalus Mangiferae samples from different areas of Pakistan cities are listed in the Table 1.
Table 1. Populations of mango bark beetles, Hypocryphalus Mangiferae samples [24]

2.5. Bactrocera zonataSaunders (Diptera: Tephritidae)
Mangoes are severely harmed by insect pests (approximately 492 species), including hoppers, flowering midges, fruit borer, mealy bugs, fruit bugs, and a wide variety of other insects. For horticultural crops, fruit flies really are a significant problem [29]. Fruit flies are the main cause of economic loss almost in every horticultural crop worldwide, resulting in fruit losses in both quantity and quality [30]. The genus Bactrocera is reported to include around 400 species, which may be located in Asia, Australia, Africa, and the Pacific region [31]. The most prevalent species in Pakistan are B. zonata, B. cucurbitae, B. dorsalis, and B. cucurbitae [32]. 30-80% of horticultural crop losses are caused by fruit flies [33], with a yearly damage across Pakistan of $1.3 billion.
In certain mango-growing regions where no preventative steps were taken to reduce the insect infestation, 100% damage is expected [34]. Fruit fly damage surpasses 80% and is rising with each passing year. Therefore, the main objective among all control efforts is to eradicate or drastically diminish fruit fly behavior [35].
Numerous fruits are affected by B. Zonata infestations, including apricots, guavas, even peaches (EPPO, 2005; Ghanim, 2009). It now impacts a wider variety of economic crops, including mango, apple, citrus, and numerous others [36]. Due to fruit fly assaults on agricultural products, notably mangoes, the European Union (EU) is endangering Pakistan's exports. The EU has previously rejected more than 200 shipments of fruit with fruit flies. In response, the EU handed Pakistan a yellow label for mango shipments and threatened that 5 further deliveries of mangoes contaminated with fruit flies will result in the cessation of Pakistan's supplies to the European nations (EU, 2104).
Agricultural yields are controlled and enhanced through the use of pesticides. The foremost portions of all pesticides applied globally are insecticides (5.2 billion pounds). Although using pesticides to control insects is the easiest method, this method comes with major drawbacks. Around 3 billion farm workers are poisoned with hazardous pesticides, which lead to the deaths of 18000 individuals, according to the World Health Organization UN Environment Program.
2.6. Toxicity of different insecticides against fruit fly, Bactrocera Zonata Saunders (Diptera: Tephritidae)
Bacterocera zonata (Saunder) (Diptera: Tephritidae) is a prominent fruit pest in Pakistan, causing significant losses in fruit yield and quality. Its management has depended heavily on the indiscriminate and injudicious application of conventional pesticides, which has resulted in insecticide resistance, environmental degradation, fruit taint, and health problems. The primary purpose of this study was to compare the efficiency of a bio-insecticide, Emamectin benzoate, to that of other commonly used insecticides against B. Zonata. After 24 hours of treatment, the findings indicated that Emamectin benzoate had a high toxicity with an LC50 value of 38.25, followed by Trichlorfon, -cyhalothrin, and Imidacloprid, which had Lc50 of 44.21, 58.98, and 187.81ppm, respectively. Based on our findings, we believe Emamectin benzoate is an efficient and ecologically safe alternative to other conventional pesticides used to control B. Zonata.
- Insecticides
The pesticides' contact residual toxicity revealed various degrees of toxicity. The LC50, LC90, and toxicity percentages of several insecticides versus B. Zonata adults are presented in Table 2. Insecticide toxicity against adult B. Zonata is ranked as follows: Emamectin benzoate > Trichlorfon > -Cyhalothrin > Imidacloprid. Among the pesticides tested, Emamectin benzoate showed that it was the most powerful. It is a semi-synthetic abamectin derivative created for the controlling of lepidopterous insects on vegetables all over the world [37]. It increases the release of neurotransmitter-aminobutyric acid that causes irreversible paralysis and death in the target invertebrates. Furthermore, it has translaminar action, which is typical of systemic insecticides. Emmamectin-treated leaves maintain a pool of active component, resulting in pest control as a consequence of larval feeding [38].
Table 2. Toxicities of Pyrethroid insecticides against Droschica Mangiferae
Emamectin benzoate degrades quickly on the plant's surface resulting in minimal exposure to beneficial substances [39].
In a previous study, a bio-insecticide compound was shown to be efficient against B. Zonata at extremely low concentrations [40]. Our experimental outcomes are consistent with theirs. Tricholofon and -cyhalothrin are somewhat effective, raising concerns about the development of pesticide resistance against B. Zonata populations. A previous experiment revealed a high degree of Trichlorofon resistance and a modest level of -Cyhalothrin resistance. In their trial, the degree of resistance maintained at 1.00-41.82 times with Trichlorfon and 1.07-18.24 factors for -Cyhalothrin [41].
Among the pesticides tested, Imidacloprid showed that it was the least efficient. It is possible that this is owing to its widespread method of action and minimal residual toxicity. There are contradictory reports of Imidacloprid efficacy in the literature. In a study, Imidacloprid was shown to have an LC50 value of 211ppm with a low degree of resistance [42]. However, other studies suggest that Imidacloprid produces good outcomes [43].
3.1. Toxicity of nanoparticles against Bactrocera Zonata as bio-control agent
Mango is a fruit with significant commercial value. Pakistan ranks fourth in mango both exports and production. Annual losses of US$200 million are caused by insect attacks. Fruit fly is among Pakistan's most serious insect problems. These are often regulated by the employment of very toxic pesticides with an adverse effect on the environment on non-target creatures. Better methods for controlling fruit flies were investigated in the current study. Metallic nanoparticles are being employed in a new strategy for controlling Bactrocera Zonata to reduce the negative impacts of pesticides on living health. Adolescents of B. Zonata have been obtained from contaminated fruits in Multan mango plantations for this study. Up to 7 days, mortality was found. The highest death rate was recorded at 160 ppm (greater concentration), whereas the lowest mortality rate was reported at trace amount (20 ppm). Higher concentrations found to be more useful for control purposes. As a consequence, total mortality is 70-80%, resulting in effective bio-control with reduced detrimental impacts on the environment and livings.
- Mycotoxins Toxicity from Mango
Mycotoxins are naturally occurring fungi-produced chemicals that are hazardous to both animals and humans [44-45]. Mycotoxins are thought to infect around 25% of the world food crops each year [46]. Patulin Mycotoxin (a Polyketide lactone 4-hydroxy-4H-furo (3,2c) pyran-2 (6H)-one (Figure 2); [47-48] is a low-molecular-weight hazardous chemical (154.121 g/mol) [49-50]. Patulin (PAT) has the chemical formula C7H6O4 and is stable in water-based environments at 105-125 °C with a melting temperature of 110 °C. It is a crystalline, colorless substance [51-52]. Because to contamination with fungus species including such Penicillium expansum, Aspergillus clavatus, and Byssochlamys nevea, PAT is frequently connected with fruits, beverages, and derived products, particularly meals meant for young children [53].These fungi that produce Patulin target vulnerable items throughout growth, harvesting, preservation, or food manufacturing. Penicillium expansum, which would be found in many different types of fruits, is the most prolific generator of PAT [54-56]. Patulin has always been connected with apples and apple-based goods. The poison, however, might infect other fruits, rotting feed, uncooked beans, and wheat bran residue. It has been proposed that cold places may become vulnerable to temperate difficulties involving Patulin.

Figure 2. Structure of Patulin Mycotoxins [69]
PAT has a vital act in the agriculture zone and food business because of contamination of feed and food at all processing stages, storage, transportation, and sale. When infected fruits and derivative products are consumed, PAT Mycotoxin leads to health issues. PAT toxicity is caused by the production of harmful addition reactions containing Sulfhydryl groups, which causes acute and long-term toxicity concerns in both animals and individuals [57].
This Mycotoxin has been linked to immunologic, neurologic, and digestive consequences which including dilatation, ulceration, and bleeding [58-59]. PAT may affect the kidneys, liver, intestine, spleen, and stomach. Among mammalian cells and animals, PAT toxicity comprises Mutagenicity, Teratogenic effects, Embryo toxicity, and hypersensitivity [60-61]. PAT is classed as "not classifiable as to its carcinogenicity to humans" by the International Agency for Research on Cancer (IARC) [61]. Because of the negative health effects of PAT, safe values of PAT throughout foodstuffs have been established. The maximum amount of PAT for fruits and beverages set by the Codex Alimentarius is 50 g/Kg [62].
The European Union (EU) established maximum amounts of PAT in fruit drinks (50 gram/kg), compact apple products (25 g/Kg), and meals targeted for babies and young children (10 g/Kg) in Commission Regulation (EC) No. 1881/2006 [63]. Countries such as China, the United States, and Canada have also set the maximum limits of PAT in foods, especially in apple-based products, ranging from 25 to 50 g/Kg [64-65]. In addition, a Joint Expert Group on Food Additives has defined a preliminary maximum tolerated daily consumption of 0.4 g/Kg body mass [66]. Because apples and apple-oriented products are well recognized as the primary sources of PAT in the human diet, the bulk of documented investigations involve Patulin detection in apple-based foodstuff [67-68]. PAT monitoring in plenty of other fruits as well as fruit-oriented products and on the other hand, should not be overlooked. A recent investigation on various fruits, juices, and smoothies in Pakistan demonstrated the presence of PAT in further than 50% of samples, with a concentration range of 182 gram/Kg (Iqbal et al. 2018) [69]. Mango and orange fruits, as well as their derivatives, were not included in the poll. HPLC chromatograms of natural occurrence of Patulin in mango sample (A), as demonstrated in Figure 3.

Figure 3. HPLC chromatograms of natural occurrence of Patulin in mango sample (A) [69]
- Toxicity by Means of Three Synthetic Insecticides in Controlling Mango Hopper, Idioscopus Clypealis
The reduction in population size suggested that all of the pesticides tested were effective against the mango hopper. Imidacloprid and Endosulfan had the greatest percentage reduction across pretreatment in contrast to the control plot, followed with Cypermethrin, but also Neem oil. As far as overall effectiveness was concerned, there was a significant difference (P< 0.05) between the therapies. In the case of conventional insecticides, Imidacloprid was found to be highly effective with 83.63% reduction after 24 hours of first spray with an increasing trend in efficacy as 89.97% after 72 hours. However, its efficacy reduced at 168 hours up to 85%, so although Endosulfan confirmed to be an excellent controlling agent with 75.79% reducing at 24 hrs with such a gradual decrease after 72 and 168 hrs of sprinkler with 72.48%, but also 67.47% reduction, Cypermethrin was less efficient than Imidacloprid and Endosulfan, providing 64.40% decrease after 24 hours, increasing to 68.23% reduction after 72 hours, and 57.95% reduction after 168 hours of initial spray. Similar findings were reported by, who found Imidacloprid to be the most effective insecticide versus okra jassid (Amrasca biguttula biguttula). The effectiveness of various dosages of Imidacloprid as a seed plus root treatment agent against Chilli thrips was evaluated and shown to be beneficial up till 45 days after treatment. When compared to Endosulfan and Neem-Oil, Imidacloprid was shown as the more powerful towards mustard aphid, whereas Endosulfan was discovered to be particularly efficient towards jassids on Okra plant. Endosulfan and Cypermethrin were tested for aphid control on Okra and Brinjal crops at various time intervals and it was discovered that Endosulfan is more efficient over Cypermethrin upon Brinjal harvests and vice versa on Okra crops.
The impact of Endosulfan and Azadirachtin on beetroot shoot and fruit borer was tested, and azadirachtin was shown to be somewhat successful when used alone, but it varied in efficiency when used in combination, including such Endosulfan + Bt (Bacillus subtilis), and Azadirachtin + Bt. In any event, Azadirachtin was proven to be less efficacious than Endosulfan. Whereas comparable pesticide combinations with Cypermethrin demonstrated technical performance of certain pesticides over others against okra aphids and Jassids, Cypermethrin had shown modest but still significant dominance [70].
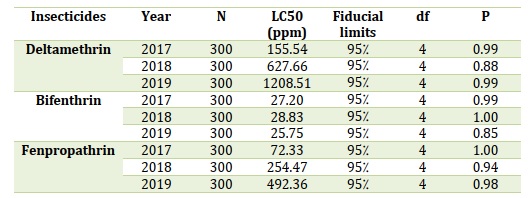
5.1. Toxicity of pyrethroids
Deltamethrin, Bifenthrin in addition to Fenpropathrin toxicity over mature female mealybugs throughout three years (2017-19). Overall, Bifenthrin toxicities were substantially greater (95% FLs did not intersect) than Deltamethrin toxicities from 2017-19 and Fenpropathrin toxicities from 2018-19, although Bifenthrin toxicities from 2017-19 were comparable (95% FLs matched) to Fenpropathrin being tested in 2017 and compared, as indicated in Table 2 [70].
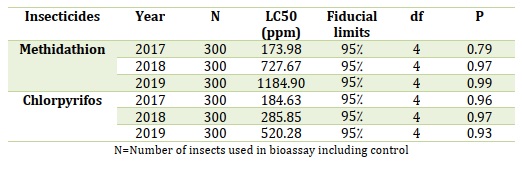
5.2. Toxicity of organophosphates
The hazards of organophosphate pesticides, Methidathion, and Chlorpyrifos studied in 2017-19. Methidathion displayed comparable mortality (95% FLs coincided) throughout all 3 years (2017-19), as provided in Table 3 [70].
Table 3. Toxicities of organophosphates insecticides against Droschica Mangiferae
5.3. Toxicity of neonicotinoids
In the neonicotinoid category, three insecticides, imidacloprid, acetamiprid, and thiamethoxam were investigated against further field collected D. mangiferae to assess their toxicity from 2017 to 2019. Toxicity of imidacloprid continued statistically comparable (95% FLs covered) from 2017 to 2019, with LC50 values of 112.71 ppm (95% from 2017 to 2019), thiamethoxam toxicities were considerably greater (95% FLs never coincide) than Acetamiprid toxicities and Imidacloprid toxicities, as demonstrated in Table 4 [70].
Table 4. Toxicities of neonicotinoids insecticides against Droschica Mangiferae

5.4. Toxicity of tetramic acid derivative and pyridine insecticides
Among some tetramic acid derivatives as well as pyridines, spirotetramat, or flonicamid were investigated for toxicity against D. mangiferae from 2017 to 2019. Consequently, spirotetramat and flonicamid demonstrated toxicities similar to bifenthrin (95% FLs overlapped), but much greater (95% FLs could not overlap) than any other examined (Table 5) [70].
Table 5. Toxicities of tetramic acid insecticides against Droschica Mangiferae

- Alternaria Alternative Disease's Effect Upon Organic Constituents of Mango Leaf Tissue
Mango is afflicted with various illnesses at all earlier stages among its existence [71]. Almost no plant organ is immune; nearly every portion, along with the roots, twig, leaf, stem, petiole, floral, and fruit, is infected by a huge variety of diseases [72]. However, there are just a few illnesses that are economically significant. Such diseases appear as rot, necrosis, dieback, mildew, leaf spot, canker, scab, wilt, and deformity [71]. Alternaira-spp is recognized to generate toxin, which is an essential component of pathogenesis [73]. The poison travels fast through the circulatory system, producing electrolyte leaks and necrotic ulcers anywhere along veins. As a result, Alternaia infection may result in lipid per oxidation, (H2O2) production and sometimes even cell death. As a result, during the infection course, the fungal toxin has a significant impact on the plant's photosynthetic network as well as associated physiological and biochemical features [73-75].
The organic components of healthy and sick mangoes (Mangifera indica) stems were studied, including chlorophyll, sugar, ascorbic acid, proteins, and Carotenoids. The infection from Alternaria alternate (Fr.) Keissl severely harmed most organic constituents in mango leaves. Chlorophyll, ascorbic acid, Carotenoids, protein, and sugar levels were discovered to be reduced, whilst 20.27%, 14.06%, 32.86%, 31.73%, and 18.02% in infected mango leaves, respectively. The quantities of ascorbic acid as well as proteins revealed discovered to be considerably afflicted.
- Toxicity Due to Artificial Ripening
7.1. Ethylene ripening
Ethylene is a phytohormone, which is a naturally produced plant hormone. The mechanism through which ethylene stimulates ripening is currently being studied. Nonetheless, it is clear that fruit ripening is accelerated to ripen in the presence and influence of ethylene. As a result, post-harvest ethylene treatment is utilized to enhance ripening in green vegetative growth. The chemical ethephon that metabolises into either ethylene inside the fruit is used for commercial applications of ethylene. Ethephon is regulated equally by EPA and FDA. According the National Insecticide, Rodenticide Act (FIFRA), and Fungicide, EPA provides regulatory control of application mostly during harvest, whereas FDA has regulatory control of residue on food products under the Food Safety and standards act (FFDCA). Nevertheless, ethephon is just not regarded a hazardous chemical, and therefore no specific tolerance for usage mostly on mangoes has been established. Furthermore, FDA has classified ethephon as generally recognized as safe (GRAS). Ethephon demonstrates non-obvious detrimental repercussions when utilized for the reasons intended and in compliance with acceptable manufacturing technique [76].
7.2. Calcium carbide ripening
Green mangoes may be swiftly ripened using calcium carbide, a cheap, and commonly accessible industrial chemical. Ripening demands solely that green fruit be here in close proximity to either calcium carbide. Small packets or canisters containing calcium carbide are essentially inserted in the box or vehicle conveying unripened mangoes. When calcium carbide needs to react with moisture in its surroundings, it generates acetylene gas, something that is indeed comparable to ethylene and though significantly extra toxic. Although acetylene gas functions similarly to ethylene as just a souring agent, the residual of its consumption has severe negative health consequences. Acetylene is thought to have an effect on the neurological system via decreasing oxygen delivery towards the brain. Moreover, arsenic as well as phosphorus, all these hazardous chemicals, is present in industrial-grade calcium carbide. The World Health Organization (WHO) lists arsenic as a toxin, as well as FDA has mentioned that continuous exposed to arsenic potentially lead in cancer. White phosphorous, which is linked with industrial-grade calcium carbide, is an extremely hazardous chemical. White phosphorous is "highly harmful to humans," according to the EPA, and short-term exposure can cause "rapid drop in condition accompanying gastrointestinal consequences, as well as serious consequences mostly on kidneys, cardiovascular system, liver, and nervous system" [76]. Consumption of calcium carbide-ripened fruits has been linked to stomach discomfort, persistent hypoxia, hypertension, dizziness, tiredness, trouble concentrating, loss of memory, cerebral edoema (brain inflammation induced somewhat by surplus fluids), and migraine [77]. Despite its legality, calcium carbide is now employed inside the mangoes maturation across Brazil, Malaysia, Costa Rica, India, Senegal, Pakistan, Philippines, in addition to South Africa [76].
- Problems Pertaining to Mango Micropropagation
Micropropagation requires a robust in vitro culture beginning since failure at this point puts a tissue culturist in a hopeless situation with no hope of recovery. A number of inherent issues with mango in vitro culture, including phenol evaporation, moderate discoloration, browning of explants, systemic contamination at a deep level, and in vitro recalcitrance of tissues, have been clearly demonstrated in preliminary studies. These issues, either individually or collectively, put the entire tissue culture effort in jeopardy.
8.1. Explants necrosis, medium browning, and phenol exudation
An effective regeneration process should be developed in order to enhance mango harvests using biotechnological techniques such as genetic manipulation, in vitro mutation accompanied by selection, and restoration of superior phylogenetic analysis variants through cell, protoplast growth, etc. Compared to the majority of horticulture crops, mango has proven a challenging crop to manage. There have been several attempts to rejuvenate mango using explants of leaves and shoots [78-79]. Although these techniques were discovered to be ineffective, they nonetheless guarantee the year-round availability of explants, unlike the explants offered by the immature fruit lets, which are only available at specific times of the year [80-81].
For workers handling this crop, however, the high Phenolic exudation brought on by activation of the oxidative enzyme system during plant excision, explants browning, deeply ingrained contamination, medium discoloration, and poor in vitro growth performance have made it challenging [78]. Suggested preparation of leaf extract adopting liquid shaker culturing to lessen phenolic-volatilization throughout in vitro propagation. Explants were maintained in 1% PVP-supplemented liquid MS media before being inoculated into gelled MS medium. Later, Chavan et al. (2000) provided another method to lessen explants browning: immersing apical and auxiliary branches in % sucrose and 0.5% polyvinylpyrolidine (PVP) + for 30s. In vitro cultivation of etiolated mango shoots showed a significant drop inside polyphenol oxidase (PPO) efficiency and phenol content, PPO activity, phenol concentration, and explants survival all correlated negatively [81]. In addition, found that etiolated mango shoots had significantly lower residual phenol, peroxides, and PPO activity than non-etiolated regulate mango shoots. Since etiolated shoots had decreased cumulative in cultured cells phenol exudation during culture, they further experienced much greater survival and less necrosis than control [82].
8.2. Undiscovered systemic contamination
The existence of deep-seated contamination, which commonly causes cultures to fail, is one of the most significant inherent limitations for culture initiation utilizing vegetative plant components. As latent endophytic pollutants may not immediately manifest themselves in the form of apparent growth on the plant parts or culture medium, they may later cause problematic for the culture or may be passed to a plantlets that are formed. The infection could require developing in vitro or it might be unable to spread till the culture is moved to a mid that is more suited for its growth. Alternaria-tags, Fusarium subglutinans, Colletotrichum sub sp., Dothiorella species, and are four dormant fungal infectious diseases reported in mango [83]. These are observable inside the shoot tips and within separate components of such panicle even in healthy cells, although with lower density [84]. These latent pollutants may be found in plant tissue's cortex, phloem, and xylem, including Parenchymatous pulp cells as well as intra- and intercellular spaces. They reduce the membrane, which affects the culture's ability to survive. Even additional disease structures inside host tissues. Although Chandra, Ravindra and Thomas [85-86] attempted to totally eradicate pervasive endogenous contamination, they were unable. Mango aseptic cultures might be maintained using particular antibiotics against impurities if endophytic organisms can be isolated and identified. To lessen the contamination burden, Hare Krishna [87] recommended applying imidazole sprays over field-grown stock crops three times at intervals of three days. Sprays with bavistin and streptomycin administered alternately at intervals of two days performed better than sprays with only Imidazole. In addition to decrease infection, imidazole spray postponed the appearance acute necrosis inside shoot tip cultural contexts for monoembryonic mango cultivars Amrapali or Pusa Arunima [87].
8.3. In vitro recalcitrance
Tissue culture is indeed known as recalcitrance. Three key elements; namely, the donor's "whole plant" physiology, in vitro manipulation, or in vitro stress causes, have a significant impact on tissue culture outcomes. Explants from donor plants that are robust and well described should be collected. However, there may not be as many options for donor plants, particularly in the case of the mango, where effective regeneration was only seen in premature early embryonic Nucellar embryos/(juvenile vasculature), while other explants like adventitious roots, nodal segments, leaves (mature tissues), etc. showed varying degrees of success. The embryonic and nuclear tissues' youth is the cause. Nevertheless, the genotype has a significant impact on success. However, growth state of donor plants, as well as the growth level of embryo on separation, is of utmost significance. Recalcitrance is indeed the reluctance of plant tissue, tissues, and perhaps organs. Occasionally, using mature tissues is unavoidable since explanting inside the former scenario depends on a small window of time during the year when ripe fruits reach the right state now for explanting their nucleolus.
At every stage of cultivation, mature explants show recalcitrance. To overcome resistance in these systems, the choice of explants during a certain sensitive phase of such a mature massive tree life span is crucial Thomas and Ravindra [85] recommended using nodal segments from the present season especially shoots for improved survival, little phenolic exudation, and growth response based on their findings. Yang and Ludders [88], on the other hand, were successful in growing new shoots from shoot tips. The parent plants were kept in glasshouse conditions, and May-June explanting produced the best results when compared to the other months. Recalcitrance modulation is greatly influenced by in vitro manipulation. Recalcitrance can be reduced by manipulating many components, including inorganic, amino acids, organic, phytohormones, enzymes, carbon sources, gelling agents, and other media supplements. Optimizing the plant development control regime is among the most crucial solutions to this issue Raghuvanshi and Srivastava [78] created countless mango branches from callus obtained from adult leaf extract by combining several Auxin and Cytokinin compositions, such as NAA, BA, IAA, and kinetin for Ms Media.
The light regime and amongst vitro plant stressing physiology are two additional significant parameters that have an impact on the intransigence of mango tissues. As previously mentioned, a few employees have recommended preserving mango culture in the dark. Due of their significant phenolic contents, explants on cultivation undergo oxidation, which is the most significant in vitro stress factor. In mango, which has a massive concentration of phenols linked to larger ongoing and lignification, it is more common than in many other crops. A wound reaction in cut explants is the degradation of phenolic chemicals, and culture procedures like surface sterilization may further increase this [87].
8.4. Future thrusts of micropropagation
Mango enhancement presents many prospects for biotechnology. To raise homozygous lines, tissue culture procedures such as another and ovary growth can be employed. Similarly, genetic modification to produce persistent transforms ants for various traits are progressively being investigated. In Mangifera spp. genetic markers are particularly important since they can help with traditional breeding techniques. Several mango cultivars have shown significant advancements in the creation of regeneration methods. It has also been effective to transform mango by repeated somatic embryogenesis.
Despite the effective regeneration of several genotypes, somatic embryos seldom develop into healthy plantlets. Enhancing the frequency at which somatic embryos develop into healthy plantlets and regenerating planting material via shoot/nodal divisions should be the main goals of future research.
Large canopies prevent the majority of significant mango varieties, such as Haden, Alphonso, Kent, Sensation, etc. from being incorporated into the idea of higher densities planting. These varieties dominate the global mango trade. Therefore, the occurrence of alternating bearing is yet another significant issue that complicates the development of several mango types. AGL20 integrates signals from several pathways incorporating both internal and external stimuli to play a crucial role in flowering evocation [89].
Similar to how biotechnology has advanced, it is now capable of overcoming both biotic and Abiotic stressors. The generation of changed variety(s) meant to suit the specified goal by exact genetic modification, which was heretofore impossible through traditional breeding, is just a larger potential of biotechnology to transform the mango business [87].
- Heavy Toxic Metals
Fruits include essential nutrients for maintaining physical and mental well-being [89]. Fruits are an excellent source of nutrients and vitamins [90]. Several nutrients, including fibre, potassium, sulphur, magnesium, phosphorus, calcium, copper, and thiamin, are primarily found in fruits. Niacin, thiamine, vitamin b, nicotinic acid, vitamin B6, minerals, Vit C, other flavonoids were included in the investigation of the toxic content of heavy metals [91-92]. Citrus fruits are therefore extremely high in vitamin C [93]. Unfortunately, humans lack the capacity to synthesize vitamin C, while other animals, like fish and reptiles can generate vitamin C in inner livers.
As a result, it is necessary to get enough vitamin C via diet [94]. Furthermore, vitamin C seems to have a reputation for being a potent antioxidant. Chemical substances known as antioxidants prevent oxidation. In addition, antioxidants stop the production of extremely reactive oxygen compounds (ROS). Deoxyribonucleic acid and bio-molecules may be destroyed by the elevated ROS (DNA) [95]. Moreover, include fruits regularly in your diet lowers your chance of developing chronic conditions including cancer and cardiovascular disease [96]. Regular consumption of potassium-rich foods lowers the incidence of kidney disease, strokes, cardiovascular disease, and cancer [97].
Nevertheless, increased fertilizer and pesticide use as well as the utilization of industrial effluent for the development of fruit trees might raise the amount of heavy metals of fruits [98]. Because pesticides and industrial effluent are the major sources of hazardous heavy metals, numerous illnesses, including cancer, coagulopathy, hematemesis, liver damage, hematochezia, immunotoxicity, breathlessness, melena, cardiac collapse, renal, and bone problems are among the numerous illnesses that heavy metals may produce [99]. Heavy metals have also been related to disorders including Parkinson's and Alzheimer's [100]. In addition, heavy metals may start the excessive ROS production that is the primary factor in DNA and bio-macromolecules damage [101].
Several heavy metals sometimes referred to as micronutrient; seem to be necessary for healthy bodily development and expansion at low concentrations. Thus, the need for insulin is decreased when chromium (Cr) is present in the blood. Furthermore, Cr works with insulin to help make proteins, whereas a Cr deficit impairs glucose control [102]. The creation of hemoglobin and proper operation of vitamins, regulators, and enzymes both depend on cobalt (Co) [103] Nickel (Ni) seems to be a crucial trace mineral for eukaryotes, native plants, and microorganisms. Several important metalloid-enzymes' activity sites require Ni to function properly [104]. Lead (Pb) as well as cadmium (Cd) is two non-essential contaminants that are widely known to have harmful effects on people [105].
Fruit drink (juice) is a rich provider of antioxidants which prevents excessive ROS from being produced, but heavy metals are the ones that cause such organisms to be produced. It is possible to draw the conclusion that fruits' excessive heavy metal concentration may diminish their antioxidant capacity. To determine the presence of the most hazardous heavy metals, including such Cr, Ni, Cd, Co, and Pb, in the most consumed fruits inside Khyber Pakhtunkhwa and Punjab Pakistan (mango, banana, guava, lemonade, peach, apple, Cherry, and grape) research study examined these fruits (Table 6).
Table 6. Mean concentrations (mg kg–1) of toxic Heavy Metals in fruits on dry weight

In addition to determine environmental or ecological relationships between trace elements, the current investigation concentrated on determining passive elements levels within various regions of three distinct categories of samples. A key component of human diets, mango fruits is really a summertime seasonal crop that grows on land used for farming and contains high quantities Cu, Iron, and maybe even Zn. On the premise of diversity and area, the examined samples were divided into categories. The average findings of three different samples for Lead, Cadmium, Copper, Iron, and Zinc as determined using the suggested method for determining metallic ions. Low amounts of Pb (5.280.28), Cd (2.020.11), and Fe (0.8420.02) were detected in Shujabad, Tandoallah Yar, and Multan, respectively, whereas low concentrations of Cu (0.6970.04) in addition to Zn (1.010.05) were discovered in Muzfar-Garah and Sadiqabad, as given in Table 7.
Table 7. Elemental contents in mango fruits of different region of Pakistan [106]

Mango fruits had 0.697 through 1.82 mg Kg-1 of such copper (Table 7). There was no discernible difference in this value across the various research regions. It is interesting to notice that whereas Muzfarghar had low levels of Cu, Hyderabad had greater levels. Not only Rahim-Yar Khan, but also Multan both of them have excessive levels of iron (2.710.18) and Zinc (2.720.18) mg Kg-1, correspondingly (Table 3). A healthy metabolism depends on the mineral zinc. Comparing the collected results at a 95% confidence level revealed the major variances across the various areas of Pakistan. In this study, Multan, Sahibabad, and Muzfarghar were shown to have lower levels of Iron, Cu, and Zinc (Table 7). On the other hand, Pb and Cd concentrations were greater in majority of the examined localities and greater inside the Dushari type, respectively.
Furthermore, in 8 samples, Pb levels were found to be greater in the Langra (L) variety, with Chaunsa in addition to Dushari (C) obtaining the second and third highest levels of Pb, respectively. According to EU standards, the Pb concentration of mango fruit gathered from various places was determined to be inside the allowable range (Table 7). Rahim-Yar Khan had the greatest average content of Cd around 5.73 0.33 mg Kg-1, while Khanewal had the highest amount of Pb at 12.51 0.73 g Kg-1 (Table 7). All of the chosen varieties had a maximum Cd content of 5.73 g Kg-1, although Multan had comparatively lower concentrations of Cd as well as Pd at 3.81 0.21 g Kg-1 and 4.06 0.32 g Kg-1, respectively [106].
- Conclusion
In Pakistan's farming community, the widespread use of pesticides for mango production poses serious environmental and health risks. To better control various insect pests and illnesses, most farmers employ a combination of chemical pesticides. 70% of pesticides in use are highly or moderately harmful to the biological system. The application and handling of these hazardous pesticides without due care contaminates the air, water, and land, as well as all three types of environmental matrices in addition to discuss mango diseases (including the mango mealy bug, Hypocryphalus magiferae, Bark Ambrosia Beetles, Bactrocera zonata, Hopper, and Idioscopus.
Mango fruit cultivation plays a significant role in Pakistan's agricultural industry. Through contaminated air, trace, and harmful metals (Cd, Pb, Fe, Cu, and Zn) can enter natural water and soil, and then fruit or plants. This might lead to food chain contamination, which would be extremely harmful to people's health.
The main causes of higher pesticide residues in agricultural commodities and consequently higher risk of exposure to end users (i.e. humans) are farmers' sole reliance on chemical pesticides, lack of knowledge, and access to training facilities for farmers, and improper handling. To increase farmers' understanding of the use and correct management of pesticides, the government must impose strict rules on their manufacturing, distribution, and make training facilities and effective extension services easily accessible to the agricultural community.
Orcids
Muhammad Khalil: https://orcid.org/0009-0002-5115-0386
Shaista Noor: https://orcid.org/0009-0004-2903-2310
Zaheer Ahmad: https://orcid.org/0000-0001-6989-1620
Fawad Ahmad: https://orcid.org/0000-0003-2404-5572
Acknowledgements
This work is financially supported by Chemistry Department, University of Wah.
Citation: M. Khalil*, S. Noor, Z. Ahmad, F. Ahmad. Fate of Pakistani Exported Mango due to Its Toxicity (Heavy Metals, Pesticides, and Other Toxic Organic Components). J. Appl. Organomet. Chem., 2023, 3(2), 86-107.