Document Type : Mini-Review Article
Author
Department of Chemistry, Govt. NPG College of Science Raipur Chhattisgarh, India
Abstract
This review provides a concise overview of the past, present, and future applications of ferrocene and its derivatives in drug discovery. Ferrocene-based compounds have shown promise in various therapeutic areas, including cancer, infections, and inflammation. Despite challenges in solubility and bioavailability, recent advancements in synthesis and formulation have improved their drug-like properties. The unique redox properties of ferrocene offer opportunities for targeted therapies, while combination strategies and drug delivery systems enhance their efficacy. The emerging field of metallopharmaceuticals further expands the potential of ferrocene-based drugs. Continued research in this area holds promise for the development of innovative and effective therapeutic agents.
Graphical Abstract
Keywords
Main Subjects
Introduction
In the realm of medicinal chemistry, organometallic compounds emerge as promising contenders for anticancer drugs due to their possession of covalent metal-carbon bonds. These compounds exhibit a wide array of structures and stereochemistry, imparting the ability to regulate kinetic properties by means of ligand design. With their kinetically stable nature, lack of charge, lipophilicity, and the presence of low oxidation state metal atoms, these compounds possess desirable attributes. When compared to classical coordination metal complexes, organometallics open up novel prospects for the creation of innovative medicinal compounds that operate through distinctive metal-specific mechanisms of action. Metallocenes, half-sandwich complexes, carbene-, CO-, or π-ligands, which traditionally find utility in catalysis or biosensing, are now finding applications in the field of medicinal chemistry. Some important organometallic compounds given in Figure 1 [1, 2].
 Figure 1. Different types of organometallic compounds
Figure 1. Different types of organometallic compounds
In this perspective, we shed light on the latest advancements in the field of organometallic compounds that exhibit proven antiproliferative activity. Our specific focus is on compounds that have undergone biochemical or cell biology studies to elucidate their molecular targets and modes of action. The primary objective of this perspective is to explore the potential application of these compounds as chemotherapeutic agents against cancer. Furthermore, a recent review article classifies inorganic compounds with anticancer properties based on their specific modes of action. However, due to limitations in space, providing a comprehensive description of all types of organometallic compounds currently under investigation is not feasible within this concise review. It is worth noting that this discussion excludes a notable class of organometallic anticancer compounds known as radiolabeled organometallics. Nonetheless, Alberto et al. have conducted detailed reviews on recent developments in radiolabeled organometallic compounds, including their synthesis and applications [3, 4].
Metallocenes
Metallocenes are a class of compounds characterized by the presence of two cyclopentadienyl [Cp] ligands that form π-bonds with a central metal atom. The exploration of this compound class began in 1952 when ferrocene [Cp2Fe] was discovered, and its symmetrical structure with two equivalent, π-bonded Cp rings was determined. These compounds are often referred to as "sandwich complexes" due to their symmetrical arrangement. The term "metallocenes" is sometimes used to describe other metal complexes that possess cyclic π-systems. In addition, compounds featuring only one π-system are classified as "half-sandwich metallocenes," such as the Ru[arene] complexes discussed in subsequent sections of this perspective. Bis-cyclopentadienyl complexes can be further divided into two categories: "classical" metallocenes, characterized by parallel Cp rings, and "bent" metallocenes, which possess additional ligands bonded to the metal alongside the Cp rings (Figure 2).
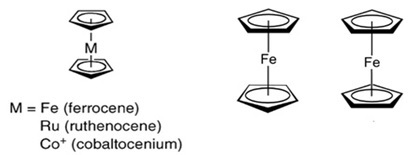
Figure 2. Classical metallocenes , staggered and eclipsed ferrocene
In the realm of medicinal applications, robust metallocenes often incorporate metals from the iron and cobalt triad, including Fe, Ru, and Co. Conversely, bent metallocenes commonly involve metals from the earlier transition metals such as Ti, Zr, V, Nb, and Mo, especially within the context of medicinal applications. Notably, all bent metallocenes of medicinal significance exhibit a cis-dihalide motif, similar to the cis-dichloro motif found in the well-known anticancer drug cisplatin. This resemblance has sparked interest in metallocenes within the field of medicinal inorganic chemistry, with notable contributions by Köpf and Köpf-Maier [5-7].
Historically, the medicinal properties of ferrocene were indeed investigated as it was the initial organometallic compound for which antiproliferative properties were reported. The discovery of ferrocene's antiproliferative activity sparked interest in exploring the potential of other organometallic compounds for medicinal applications. This early investigation laid the foundation for further research and the exploration of various classes of organometallic compounds as potential candidates for therapeutic purposes [8, 9].
Ferrocene, on its own, is not considered highly toxic. It can be administered through injection, inhalation, or oral intake without causing significant health issues. Similar to the other xenobiotics, it undergoes degradation in the liver by cytochromes. Due to its aromatic nature, it was expected to undergo metabolism similar to benzene, which was indeed confirmed through experimentation. The metabolites are subsequently excreted in urine in the form of conjugates with sulfate (a minor product) and glucuronic acid (the main product). This metabolic pathway ensures ferrocene elimination from the body [10-12].
In experiments conducted with intact liver microsomes, it was observed that the hydroxylation of ferrocene necessitates the presence of NADPH and molecular oxygen. This process was inhibited by CO when tested in vitro. However, interestingly, in vivo studies revealed that pretreatment of rats with phenobarbital substantially enhanced the hydroxylation of ferrocene. These findings serve as conclusive evidence that the hydroxylation of ferrocene is mediated by cytochrome P450 enzymes, similar to the hydroxylation of benzene and various other hydrocarbons.
Although comprehensive studies like the one described for ferrocene have not been extensively conducted on other organometallic complexes, it is reasonable to hypothesize that several metal-based drugs discussed in this article, especially those incorporating π-bonded (quasi-aromatic) ligands, might undergo analogous metabolic processes. However, it is worth noting that hydroxy ferrocene, in particular, is relatively unstable and tends to decompose when exposed to aqueous solutions, eventually liberating solvated iron atoms.
It is noteworthy that ferrocene derivatives have been suggested as potential antianemic agents, and a specific compound known as ferrocerone obtained clinical approval in the former soviet union. As far as our knowledge extends, this compound stands as the first commercially available organometallic drug belonging to the transition metal category. Furthermore, a compound containing a ferrocene moiety, closely related to chloroquine, has successfully completed phase II clinical trials as a candidate antimalarial drug. This compound is referred to as ferroquine (1), as depicted in Figure 3 [13, 14].

Figure 3. Ferroquine (1) the most advanced organometallic drug as an antimalarial drug
The ongoing field testing of ferroquine holds the potential for it to be approved as a novel antimalarial drug in the near future. Ferroquine exhibits a similar activity to chloroquine against the malaria parasite P. falciparum, but its most significant attribute is its effectiveness against chloroquine-resistant strains of P. falciparum. It has been speculated that alterations in lipophilicity, and potentially some redox activation, may contribute to the unexpected antimalarial activity of this ferrocene compound [14].
Following the achievements of ferroquine, numerous other organometallic antimalarial compounds have been synthesized and tested. However, their success has been comparatively limited thus far.
To evaluate the toxicity of ferrocene, beagle dogs were fed with doses of up to 300 mg.kg-1 per day for a duration of 6 months, and in some cases, doses as high as 1 g.kg−1 for up to 3 months. No acute toxicity or deaths were observed during the study. However, it was found that ferrocene administration resulted in significant iron overload in the liver (hepatic Fe overload).
Although the dogs experienced hepatic Fe overload, they were able to recover spontaneously without any intervention. To reduce the iron overload, large quantities of iron were removed through repeated venesection [the process of removing blood from a vein].
These findings suggest that while ferrocene administration can lead to iron overload in the liver, the dogs were able to recover without any adverse consequences. Proper management, such as venesection, can help alleviate the iron overload caused by ferrocene [15].
Ferrocene has the ability to undergo one-electron oxidation, leading to the formation of stable ferrocenium cation. This redox reaction is typically reversible for the majority of ferrocene derivatives. Simple ferrocenium salts have demonstrated an antiproliferative effect on certain cancer cells. However, the precise mechanism of action is not yet fully understood. It is believed that the anti-proliferative activity may involve interactions with nuclear DNA, cell membranes, and the enzyme topoisomerase II. Further research is necessary to elucidate the exact mechanisms underlying the antiproliferative effects of ferrocene derivatives [8].
Decamethylferrocenium tetrafluoroborate [Cp2FeBF4], which includes the pentamethylcyclopentadienyl ligand [Cp], has been discovered to possess cytotoxic properties. This cytotoxic effect has been linked to the generation of 8-oxo guanine, an initial product of DNA oxidation. ESR [electron spin resonance] and spin-trapping experiments have demonstrated the presence of hydroxyl and superoxide radicals as reactive species. In a limited number of studies, a synergistic effect has been observed between Cp*2FeBF4 and the iron-dependent antitumor drug bleomycin [16].
The cytotoxic effects of ferrocene or ferrocenium compounds may potentially target the cell membrane, resembling the peroxidation of membrane lipids observed in cases of excessive hepatic iron. For a more comprehensive understanding of the physiological chemistry of ferrocene and the antiproliferative properties of ferrocene or ferrocenium compounds, it is recommend referring a recent detailed review available elsewhere [17].
Neuse et al. have employed a fascinating strategy to enhance the cytotoxicity of ferrocene by attaching it to polymeric supports, specifically polyaspartamide. This approach aimed to improve the delivery and stability of ferrocene compounds, potentially enhancing their effectiveness in cytotoxic applications [18-22].
The underlying concept behind this approach is the recognition that improved water solubility may be a critical factor for the activity of ferrocene compounds. This notion is supported by the observation that the cytotoxicity of ferricenium salts strongly relies on the nature of the counterion. For instance, poorly soluble heptamolybdate exhibits inactivity, whereas ferricenium salts with good aqueous solubility, such as picrate and trichloroacetate, display significant antitumor activity. Hence, enhancing the water solubility of ferrocene compounds could be a pivotal aspect in maximizing their effectiveness as potential anticancer agents [8]. It is worth mentioning that smaller ferrocenyl polyamines were further investigated by Brynes et al. around the same time, but their results showed limited success. In contrast to the promising outcomes of attaching ferrocene to polymeric supports, the use of smaller ferrocenyl polyamines did not yield significant positive effects in the context of their intended applications [23]. The recent review by Neuse provides a comprehensive overview of the research conducted on polymer-bound ferrocene as potential anticancer drug. The review summarizes the advancements, strategies, and findings related to the use of polymeric supports to enhance the cytotoxicity and therapeutic potential of ferrocene compounds. It serves as a valuable resource for gaining insights into the development and applications of polymer-bound ferrocene in the field of cancer treatment [24, 25].
Various ferrocene derivatives have been extensively investigated for their potential antiproliferative properties. Researchers have explored various modifications and substitutions on the ferrocene core to evaluate their impact on cytotoxicity and antitumor activity. These studies have contributed to the identification of promising ferrocene-based compounds that exhibit potential as candidates for antiproliferative agents in the field of cancer research [26-31]. One notable example among the tested ferrocene derivatives is the ferrocene-acridine conjugate, which has demonstrated high cytotoxicity. In this compound, the acridine moiety plays a crucial role in bringing the ferrocene close to DNA through intercalation. This proximity to DNA enhances the compound's potential to interact with the DNA molecule, leading to increased cytotoxic effects and potential antitumor activity. The ferrocene-acridine conjugate represents a promising example of the synergistic combination of different functional groups to enhance the cytotoxic properties of ferrocene derivatives [29].
Wagner et al. conducted research on related ferrocenes in the context of boron neutron capture therapy [BNCT] [27]. Their study focused on examining the activity and behavior of these compounds in BNCT. One interesting finding of their research was the observation of distinct organ distribution patterns exhibited by the borylated ferrocene compounds. This information is significant for understanding the pharmacokinetics and biodistribution of these compounds, which is crucial for optimizing their effectiveness in BNCT applications. In the study conducted by Schmalz et al., they synthesized several nucleoside analogs of ferrocene [32]. A recent study reports on a novel chiral organometallic nucleoside analog incorporating ruthenocene. In this compound, alkylthymine and alkylhydroxyl groups are attached in adjacent positions on one cyclopentadienyl ring. The biological activities of these analogs were investigated in a human pancreatic cancer cell line [MIA-Pa-Ca-2] and were found to be significantly lower compared to three previously reported analogous ferrocene compounds. This observation suggests that the choice of metallocene metal atom [Fe or Ru] plays a crucial role in determining the anticancer properties of these nucleoside analogs (Figure 4). One of the derivatives demonstrated the ability to penetrate the blood-brain barrier [BBB], which is a crucial characteristic for the treatment of brain tumors. Although these ferrocene nucleoside analogs exhibited IC50 values in the low micromolar range, they were not as potent as the iron tricarbonyl nucleoside ananalogs previously developed by the same research group [33, 34]. Despite the relatively higher IC50 values, these ferrocene nucleoside analogs still demonstrated promising cytotoxic activity, suggesting their potential utility in the treatment of cancer, particularly brain tumors.

Figure 4. Nucleoside analog of ferrocene and ruthenocene
Redox activity is not exclusive to metal compounds, but is often observed with them. This characteristic makes it intriguing to establish connections between the redox properties of metal compounds and various cellular processes such as electron transfer, oxidative stress, reactive oxygen species formation, and the overall redox status of cells [26-35]. While determining the precise "redox potential" or "redox status" of an entire cell is challenging, researchers have made the initial efforts to explore the relationship between redox activity of metal complexes and their antiproliferative properties. However, the investigation into this correlation is still in its early stages.
Nonetheless, recent work by Jaouen et al. has proposed a potential mechanism where the activation of redox processes induces anticancer activity in ferrocene derivatives. This suggests that the redox activation of these compounds could play a role in their ability to inhibit cancer cell growth. Further research in this area will help elucidate the intricate connection between redox activity and the antiproliferative properties of metal complexes, particularly ferrocene derivatives [35]. By incorporating ferrocene moieties into existing molecules, scientists aim to exploit the unique characteristics of ferrocene, such as its redox activity and potential interactions with biological targets, to improve the pharmacological properties of the compounds. This approach allows for the development of new hybrid molecules with enhanced biological activities or altered mechanisms of action. The substitution of phenyl rings with ferrocene groups provides a platform for exploring structure-activity relationships and expanding the potential applications of the existing drugs and natural products [36-38].
An important discovery in this research is the identification of a group of tamoxifen derivatives, referred to as ferrocene by Jaouen's group. Tamoxifen (2) (Figure 5) is a well-established chemotherapeutic drug utilized in the treatment of hormone-dependent breast cancer patients. Its active metabolite is hydroxytamoxifen (3) (Figure 5). The development of ferrocifens (4) represents a promising approach to enhance the therapeutic effects of tamoxifen and broaden its applications in cancer treatment.

Figure 5. Tamoxifens (2) and ferrocifens (4)
The active metabolite hydroxyferrocifen undergoes rapid oxidation, leading to the formation of a quinone methide intermediate [39-45]. This intermediate becomes activated and susceptible to attack by various nucleophiles. Similarly, stable quinone methides derived from the metal-free 4-hydroxytamoxifen under physiological conditions are known to contribute to its overall toxicity and mutagenic potential by forming adducts with molecules like glutathione and nucleobases [46-50].
Based on extensive studies on structure-activity relationships [SAR] [42-49] and analyses of electrochemical properties [46, 50-52], it is hypothesized that a similar chemical mechanism applies to the activated ferrocifens. Furthermore, the treatment of cell lines with ferrocifen and its derivatives has demonstrated the generation of reactive oxygen species [53].
The redox activity of the metallocene component plays a pivotal role in providing additional biological activity that surpasses that of purely organic analogs [46, 50-53] (Figure 6). Importantly, the established redox-activation mode of action is independent of the tamoxifen-related substructure.

Figure 6. Redox activation of ferrocifens as proposed by Jaouen et al.
In recent studies, the same research group has investigated ferrocenyl diphenols and unconjugated phenol derivatives, which have shown significant antiproliferative activity. It is postulated that these compounds operate through a similar mechanism of activation and formation of analogous intermediates. These findings provide further support for the hypothesis that the observed antiproliferative effects are mediated by a common pathway involving activation and the generation of related intermediates. This work is documented in references [54, 55].
To facilitate the potential clinical application of ferrocifens, various formulation studies have been conducted. These studies have explored the use of nanoparticles [56], lipid nanocapsules [57,58], and cyclodextrins [59] as delivery systems for ferrocifen compounds. In addition, the same research group has expanded their investigations beyond synthesis and conducted preliminary tests to assess the antiproliferative activity of ferrocene derivatives belonging to different compound classes. These classes include curcuminoids [60], androgen derivatives [61], anti-androgens based on the nilutamide structure [62], indolones [63], and ferrocenophane polyphenols [64]. These efforts aim to broaden the understanding and potential applications of ferrocene derivatives in combating proliferation-related diseases.
Extensive structure-activity relationship (SAR) studies have been conducted for the bent metallocene dihalides, focusing on the halides and substitution patterns of the Cp rings [6, 10, 11, 65, 66]. Furthermore, the model studies involving amino acids, nucleic acids, proteins, and blood plasma have been performed to gain insights into the interaction of these compounds with biomolecules [67]. Among these compounds, titanium derivatives demonstrated the highest activity, with titanocene dichloride even progressing to clinical trials [68]. However, despite promising results in animal models, the clinical response did not meet expectations, leading to the recent discontinuation of titanocene dichloride trials. Challenges in formulation, including drug decomposition and low water solubility, contributed to the setbacks. Previous investigations have explored the potential modes of action, such as DNA interaction, induction of apoptosis, and topoisomerase inhibition [69-72].
Despite the structural similarities between titanocene dichloride and cisplatin, there is currently no clear evidence indicating a similar mode of action, such as DNA binding and induction of apoptosis in cancer cells [73]. Instead, it has been suggested that the Ti4+ cation in titanocene dichloride, upon complete hydrolysis of Cp2TiCl2, may bind to transferrin [73].
Notably, the aqueous Ti species have shown a stimulatory effect on hormone-dependent breast cancer cells [40]. However, from an inorganic perspective, the existence of simple hydrated Ti4+ cations in aqueous solution at pH=7 is highly unlikely, as they tend to form oligomeric species and eventually insoluble titanium dioxide.
A recent computational study focused on a benzyl-substituted titanocene (referred to as titanocene Y) sheds light on its binding to DNA. The study suggests that the Cp[R]2Ti2+ dication interacts with a phosphate group of DNA, and additional interactions contribute to the stabilization of the DNA binding [74]. However, it is important to note that this computation represents a single point calculation and assumes DNA as the molecular target. Protein targets for the bent metallocenes have not yet been considered.
Recent advancements in chemical synthesis have addressed the challenges associated with titanocene dihalides, specifically poor aqueous solubility and hydrolytic instability. Amino-substituted bent metallocenes have been successfully developed to enhance aqueous solubility [75]. In addition, ansa-titanocenes, featuring covalently linked Cp rings, have been synthesized to improve hydrolytic stability [76, 77]. Both classes of compounds have shown promising biological activity.
The Tacke group has established a versatile synthetic approach via the fulvene route to access Cp-substituted bent metallocenes, yielding unbridged and ansa-bridged metallocenes [78]. In vitro cytotoxicity tests have been conducted on various derivatives, and the p-methoxybenzyl substituted titanocene Y [6] has emerged as the most active compound [79]. Remarkably, titanocene Y has demonstrated excellent activity against renal cell cancer and pleura mesothelioma cell lines, addressing the need for effective chemotherapeutic agents for these cancers. Further investigations have included testing against freshly explanted tumors and in vivo studies using xenografted models of renal cancer, prostate cancer, and breast adenocarcinoma in mice [80-83].
Mechanistic studies, primarily focused on titanocene Y, have revealed its antiangiogenic effects, activation of the immune system, and induction of apoptosis through caspases 2 and 6 [84-86]. These properties make it a promising candidate for anticancer drugs. Building upon their work and drawing inspiration from second-generation platinum drugs, the Tacke group has recently substituted two chloride ligands on titanocene Y with carboxylate groups, resulting in equally active compounds with potentially improved pharmacokinetics [87-89]. Figure 7 provides a visual representation of these developments and compounds.
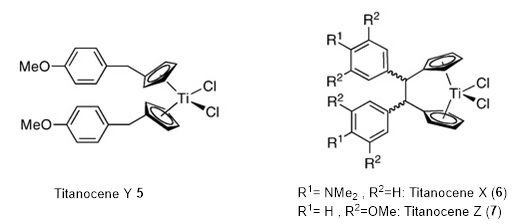
Figure 7. Titanocene Y (5) and the ansa-bridged derivatives titanocenes X (6) and Z (7)
Recent evaluations have focused on Cp-substituted vanadocenes, zirconocenes, and stannocenes [90-93]. However, molybdocene derivatives have garnered more significant attention as a promising alternative to Cp2TiCl2. Studies have identified DNA as the target, and early investigations have obtained X-ray structures demonstrating the coordination of the Cp2Mo fragment to nucleobases [94-97]. Comparative hydrolysis studies have indicated that Cp2MoCl2 is among the most stable simple metallocenes, with the Cp rings in molybdocene derivatives displaying lower susceptibility to hydrolysis compared to Cp2TiCl2.
Extensive spectroscopic investigations, primarily utilizing 1H and 31P NMR, have been conducted in solution to elucidate the binding mode of molybdocene dichloride with DNA [95, 98-100]. Recent work by Harding et al. [101, 102] has examined the cellular uptake and intracellular localization of various bent metallocene dihalides using X-ray fluorescence. Intriguingly, low levels of titanium and vanadium were detected inside cells, while significant accumulation of molybdenum was observed in the cellular nuclei. These findings provide support for the notion that different metallocenes exhibit distinct biological profiles.
Conclusion
In conclusion, metallocenes show promise as potential anticancer drugs due to their unique chemical properties and ability to selectively target cancer cells. Their ability to inhibit tumor growth and induce cancer cell death has been demonstrated in various preclinical studies. However, further research is needed to fully understand their mechanism of action, optimize their therapeutic efficacy, and evaluate their safety profile. Nonetheless, metallocenes represent a promising avenue for the development of novel anticancer treatments that may offer the improved outcomes for cancer patients in the future.
Orcids
Saroj Sharma: https://orcid.org/0000-0003-2665-7089
Acknowledgements
Author would like to thanks Department of Chemistry, Govt. NPG College of Science Raipur for providing all necessary facilities to complete this research work.
Citation: S. Sharma*. A Short Review of Past, Present, and Future of Metallocene and Its Derivatives as an Effective Therapeutic Agent. J. Appl. Organomet. Chem., 2023, 3(2), 142-155.


