Document Type : Review Article
Authors
1 Department of Applied Chemistry and Chemical Engineering, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj, Bangladesh
2 Department of Chemical Engineering, Jashore University of Science and Technology, Jashore, Bangladesh
Abstract
Furfural, an environmentally friendly alternative to petroleum-derived products, has gained significant attention as a versatile and sustainable chemical. This review article presents a critical analysis of state-of-the-art processes and technologies for furfural production from lignocellulosic biomass, focusing on bioremediation and biochemical development potential. It includes a comprehensive overview of biomass pretreatment techniques, advanced hydrolysis techniques, and highly efficient furfural synthesis methods. Recent research advances are synthesized, research gaps are identified, and future research directions are pointed out. The paper discusses various production methods including acid hydrolysis, catalytic systems, solvent-thermal conversion, and bio-refinery processes. Process steps for each approach, reaction status comparisons, and key findings are highlighted. The results obtained from the tests on parameters such as temperature, pressure, pH, and substrate concentration are consistent, with temperature being identified as an important factor affecting furfural production. In addition, this comprehensive review investigates the effects of pressure, pH, and substrate concentration on furfural production and will serve as a valuable resource for researchers, industry professionals, and policy makers involved in furfural production. It provides insight into the current status of furfural production from lignocellulosic biomass and emphasizes opportunities for process acquisition, improvement, and optimization. Ultimately, this review article will contribute to the development of a greener and more sustainable bio-based economy.
Graphical Abstract
Keywords
Main Subjects
- Introduction
Lignin, cellulose, and hemicellulose are the three structural components that make up the majority of agricultural and agro-industrial leftovers. Each of these compounds has unique properties intended for use in various chemical manufacturing processes [1]. Sugarcane bagasse produces a chemical compound called furfural (C4H3O-CHO) or 2-furaldehyde. It is an organic compound with a boiling point of 161.8 °C, is a white liquid and water soluble [2]. Xylose, a monosaccharide that is frequently present in significant amounts in the hemicellulose fraction of lignocellulosic materials, naturally dehydrates to form furfural. It is almost entirely derived from biomass. The Quaker Oats Company began producing furfural industrially in 1921 using sugar cane bagasse, corn cobs, and oat hulls, but due to low demand and expensive costs, it was never widely used. Since the 1980s or so, there has not been much of an improvement in maintenance costs, yield, or production techniques. The majority of the 300-700 kilo tons of furfural generated annually worldwide comes from China [3].
A bio-refinery is a type of conversion system that uses biomass feedstocks to create various energy carriers, chemicals, materials, food, and feed products. Furfural is a platform chemical that has the potential to replace goods made from fossil fuels in the chemical industry as an intermediary and to serve as the raw material for biofuel blends. Numerous biomass species, including bagasse, rice hulls, and corn cobs, have been investigated as potential feedstock for the manufacture of furfural. Recently, it was discovered that water hyacinth could be useful for lignocellulosic content-based sugar production [4]. The primary use of bagasse is as a fuel for boilers that provide process steam and electricity for the industry. Despite recent developments on the usage of, at the moment, acid hydrolysis of lignocellulosic (plant) biomass gives the best possibility to manufacture these platform chemicals from sustainable resources. An effective method to improve the conversion of lignocellulosic biomass is through sulfuric acid pretreatment, which may also offer economic benefits as a viable pretreatment approach [5]. Furfural alcohol can be used as the initial feedstock in various reaction pathways, as well as heterogeneous catalysts [6]. About 20% of the dry weight of rice husk is made up of inorganic chemicals, which are highly concentrated. Hemicellulose (19%), cellulose (40%), silica (17%), and lignin (16%), are the main components of rice husk. Given that rice husk contains 120 g of pentosan per kilogram of dry husk, it is possible to create furfural, an aldehyde of pyromucic acid, using this material. Furfural is used as a starting ingredient for the resins development for molded plastic and metal coatings, as well as a family of derived solvents such as furfuryl alcohol and tetrahydrofuran in various industries [7]. Pentose, which is made by the acid catalytic hydrolysis of pentosan in biomass, is converted into furfural through the process of acid catalytic dehydration. The lignocellulosic pentosan fraction is hydrolyzed by acid in batch or continuous reactors to produce monosaccharides (pentoses), which are then used to make furfural. Furfural is produced via further dehydration reactions with the pentoses. Furfural is frequently recovered from the reaction mixture using steam distillation [8]. With a carbonyl function group and a furan ring, furfural is a platform chemical that can be utilized directly or indirectly to create over 80 different compounds and fuels. Furfural can be produced synthetically from petrochemical sources, but it can also be made using environmentally benign materials like sugar beet pulp (SBP) [9].
For the manufacture of fuels, different intermediates, and end-product chemicals that are now generated from non-renewable sources, lignocellulosic biomass has been recognized as a practical alternative bio-resource. The biomass’ cellulosic and hemicellulose-derived carbohydrate molecules can be processed into various value-added goods. Mineral acids including sulfuric acid, hydrochloric acid, phosphoric acid, and nitric acid are examples of homogenous catalysts that have been widely documented to be effective catalysts for commercial furfural synthesis [10]. The conversion of biomass using catalytic thermochemical techniques has proven to be an effective method for producing a range of platform chemicals. These chemicals include furfural and 5-hydroxymethylfurfural, which are obtained in significant quantities during the conversion process [11]. Numerous physicochemical, structural, and compositional variables reduce the digestibility of the cellulose found in lignocellulosic biomass. The cellulose in the plant fibers must be exposed during the processing of lignocellulosic biomass in order to convert it to fuel. Pretreatment modifies the structure of cellulosic biomass to make cellulose more accessible. Techniques used include ammonia fiber explosion, chemical treatment, biological treatment, and steam explosion. The cellulose can then be broken down into its component sugars using acids or enzymes [12]. The complex carbohydrates found in the cellulose of plant tissues, known as plant pentosans (xylan, arabinan, and polyuronids), are used to make the solvent furfural (FF).
Due to its ability to convert relatively inert materials into useful products, the product has generated some interest. Plentiful lignocellulose feedstocks for the production of ethanol and more valuable coproduct chemicals. Currently, the industry uses product furfural for four significant and potentially significant purposes, including agrochemicals, clean fuels/biofuels, timber treatment, and PLA performance plastics [13]. Currently, only lignocellulosic biomass may be used to make furfural by dehydrating pentoses, which are normally acquired following an initial hydrolyzation stage frequently using acid catalysts e.g., acids (such as phosphoric or sulfuric acid) are employed to speed up the extraction procedure [14]. Materials high in pentosans are utilized to make furfural. Technically, the commercial production of furfural involves the acid hydrolysis of non-food pentosan polysaccharides. Food crop leftovers and wood waste. Furfural is produced via further dehydration processes of the pentoses [15]. Biomass can be converted into pellets to help address these issues while improving the pellets' physical and mechanical properties. The density (bulk or particle) of raw biomass after pelletization is increased, which lowers the handling, shipping, and storage costs. FR is a waste product created during the furfural production process using agricultural byproducts such corncob, cornstalk, rice husk, and vegetable fiber. Furfural manufacturing produces 12-13 tonnes of FR every ton [16].
Physical structure of furfural production from lignocellulosic biomass is illustrated in Figure 1.
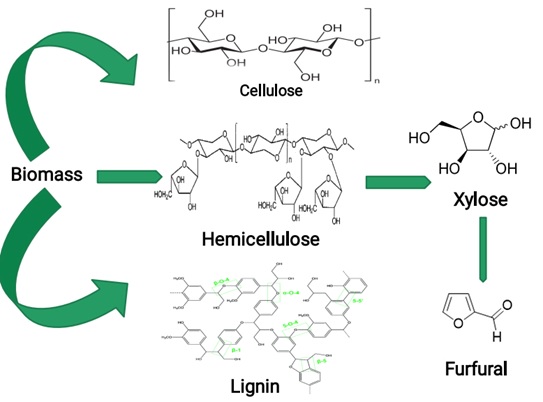
Figure 1. Physical structure of furfural production from lignocellulosic biomass
- Furfural Production
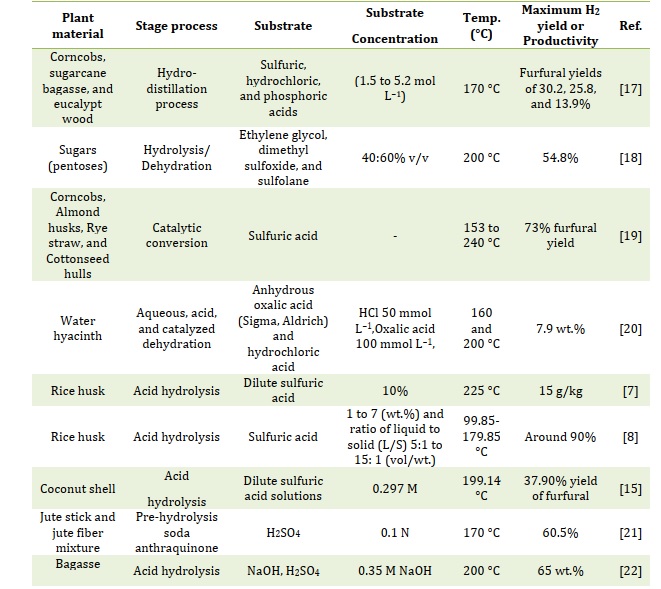
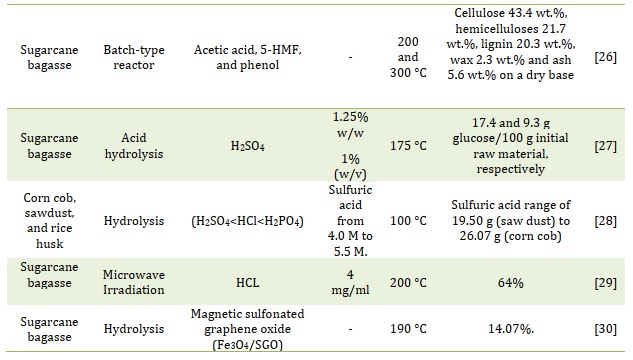
Table 1 provides information about various key parameters related to furfural production.
Table 1. Various key parameters related to furfural production

- Methods for the Production of Furfural
Different kinds of approaches are used for the furfural production from lignocellulose biomass. Some of them are described as follow in the Figure 2.
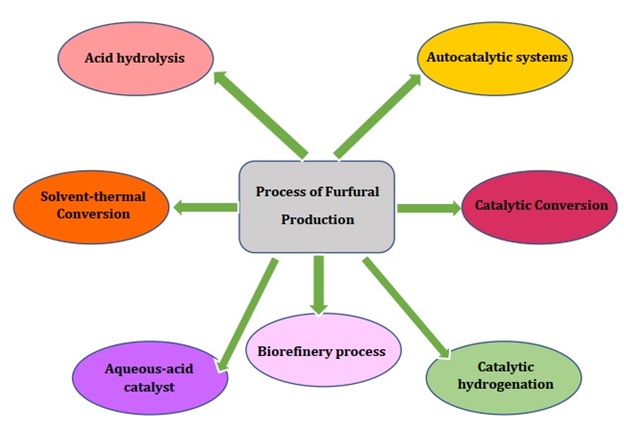
Figure 2. Methods of furfural production
3.1. Acid hydrolysis
It is possible to create furfural by depolymerizing pentosans with acid to create xylose, which is then further dehydrated to produce furfural. This preparation procedure can be carried out in two ways: in a single step where the acid cleavage of pentosans and dehydration happen simultaneously, or in two steps where the carbohydrate is initially dissolved and depolymerized under mild conditions, and then dehydrated to furfural. Hemicelluloses hydrolysis involves a four-step mechanism involving: Oxygen initially is protonated to form the connecting etheric bond, followed by the scission of the etheric bond to form a carbocation and a hydroxyl group, the reaction of the aforementioned carbocation with water, and finally, the final formation of a hydroxyl group by the intermediate deprotonation [31].
The nominal solids content for all of the trials was 8.5%. In each experiment, the reactor was filled with a mixture of 20 ml of sulfuric acid solution and 1:8 g of dry powdered olive stones. The reactor was then installed in the support and sealed. The reactor was submerged in a bath of Judaized sand that had been heated to the proper reaction temperature before the temperature acquisition process began. The pneumatic piston connected to the reactor support agitated the reacting mixture. The support was separated from the piston at the conclusion of the reaction period, and the reactor was then submerged in ice water [32].
The normal reaction process begins with the acid treatment of the biomass' hemicellulose to convert it to xylose. The liberated xylose will next undergo dehydration to become furfural. The biomass is physically pre-treated before being physically charged into a reactor and having aqueous mineral acid added to it. Steam injection into the reactor keeps the reaction at its ideal temperature. The product is continuously extracted from the aqueous stream using steam distillation. Typically, the product stream has a 3% (w/w) concentration of furfural, recovery, drying, and use of residual solids, which often comprise residual lignin and cellulose-as fuel for a boiler's production of steam [33].
The most common raw materials are bagasse, corncob, and oat husks. Three distillation columns make up the standard purification method for furfural. Initially, the reactor condensate is added to the first column (C1), also known as the azeotropic column, which contains 90% water, 4% by-products (such as acetic acid and methanol), and 6% furfural. C1 discharges wastewater at the bottom, a liquid-liquid azeotrope between water and furfural at the side, and light components at the top. Acetic acid and water waste is sometimes dumped into the sea or at a wastewater treatment facility. The second column (C2) is then fed with the top of C1 to generate methanol at its top, while C1's bottom is sent to a decanter before being recycled back into C1. A decanter naturally separates the heterogeneous azeotrope from the side of C1 into two liquid phases. To produce the required furfural, the heavy organic phase is sent to a third column (C3) while the light water phase is recycled back into the C1. Furfural is then transported to a decanter and collected at the bottom of C3, while the top of C3 is then recycled back into C3 [34].
3.2. Aqueous-acid catalyst
These methods include adding different acids to the reactor where biomass is transformed to furfural (as used on an industrial scale) in aqueous solutions. These acids include hydrochloric, phosphoric, acetic, or formic acids. Aqueous-acid catalytic systems and autocatalytic systems vary in that acid is introduced to aqueous-acid catalytic systems. Moreover, due to the more frequent occurrence of side reactions in acidic systems, by-products output is often larger in acid-catalytic systems than in autocatalytic systems. Therefore, in the methods used to produce furfural using this method, the process conditions, including temperature, time, acid, and pentose concentrations, should be carefully regulated. In this regard, furfural production was investigated in a batch process utilizing formic acid as the catalyst and commercial xylose solutions (0.067–0.20 mol/l) as the feedstock. According to the findings, raising the temperature from 120 °C to 200 °C raised the xylose conversion rate from 6.2% to 98.2%. By raising the temperature (from 140 °C to 200 °C), the furfural yield rose (from 3.0% to 65.4%); however, it declined (from 20 min to 40 min) with increasing time. Under various circumstances, the selectivity of furfural production ranged from 42.3% to 72.7% [35].
3.3. Autocatalytic systems
Biomass may be processed in batch systems at high pressure and temperature (200 °C or more) to create furfural. Acetic acid, which serves as a catalyst for the reaction in this process, is released from the hydrolysis of acetyl groups linked to biomass hemicelluloses. In addition, hemicelluloses are separated from biomass, depolymerized to pentoses and hexoses, and then concurrently transformed to furfural from the pentoses and hexoses. In one research, high temperature (180 °C to 220 °C) and 10 MPa pressure in water were used to produce furfural from commercial xylose in a batch reactor. The principal product and by-products of this procedure, respectively, were up to 50% furfural (based on xylose) and an unreported amount of formic acid, whereas xylose conversion was 95.8% at 220 °C after 50 min of reaction. Hot compressed water (HCW) was used in aqueous subcritical settings with a temperature range of 200 °C-300 °C and 200 bar to evaluate the conversion of commercial xylose to furfural. Formic and lactic acids, or byproducts, were produced during this procedure, lowering the pH of the fluid from 6.5 to 3.2. The conversion of xylose into furfural was facilitated by the high pressure, temperature, and generated acids, which would ultimately result in a more ecologically friendly procedure [35].
3.4. Catalytic hydrogenation
One of the most frequent byproducts of furfural hydrogenation is furfuryl alcohol (FA). 62% of the annual worldwide furfural production is thought to be transformed into FA. The production of foundry resins, where they are frequently formed from cross-linked polymers of FA with itself and other products (furfural, formaldehyde, phenolic compounds, urea, etc.), is where FA finds its greatest use. The resins that were produced were found to have outstanding chemical, thermal, mechanical, and corrosion- and solvent-action-resistance qualities. Furfural alcohol (FA) has been employed in the production of furan fiber-reinforced plastics for use in pipelines due to its anti-corrosion qualities, and it is further advised for high performance chemical processes when chlorinated aromatics and oxygenated organic solvents are used. Over the past few decades, many catalyst types and cutting-edge techniques have been investigated for the generation of Furfural alcohol (FA) from the furfural hydrogenation [36].
3.5. Solvent-thermal conversion
Solvent-thermal reactions, generally in the presence of aqueous solvents, organic solvents or ionic liquids have been proposed as one of the most efficient approaches to improve selectivity of chemicals from biomass. Compared with pyrolysis methods, solvolysis exhibited many advantages, such as: (i) the presence of solvents diluted the concentration of the products, thus preventing the reverse reactions and cross-linked reactions, (ii) the fluid system makes it simpler for heat and mass to transmit, and (iii) a relatively low temperature (requiring less energy) was required. Through reaction rate, reaction routes, product distributions, and yields, solvents have a considerable impact on the conversion of biomass. Therefore, by merely altering the solvent medium, reaction rates and product selectivity may be somewhat regulated.
Furthermore, fractional conversion of biomass may be accomplished in solvent-thermal procedures since the three primary components in biomass demonstrated varying solubilities in various solvents. As a result, the use of solvents in the biomass conversion may help to selectively dissolve and convert hemicellulose in the biomass to furfural [37].
3.6. Catalytic conversion
A typical catalytic experiment involved loading the stainless-steel reactor with a combination of xylose (0.1 g), catalyst OMC-SO3H (0-0.08 g), and 5 mL of solvent. Following the application of 3 MPa N2 pressure, the reactor was heated to a predetermined temperature of 160-220 °C and maintained for 0-90 min. The reactor's stirring speed during the reaction was 1000 rpm.
Following the reaction, the reactor was rapidly cooled to ambient temperature in cold water that was flowing, and the reaction mixture was filtered using a 0.22 m nylon membrane. Ultraperformance liquid chromatography (Waters Acquity UPLC H-Class) was used to examine the collected liquid after diluting it with ultrapure water and using an external standard procedure. A sugar column (SHODEX SH1011) and a refractive index (RI) detector were included in the UPLC setup. The sugar column and RI detector were both adjusted at 35 and 50 C, respectively. The mobile phase was an aqueous solution of H2SO4 (5 mM) flowing at a rate of 0.5 mL/min. The following formulas were used to compute the conversion furfural yield:
Furfural yield (%) = moles of furfural produced /moles of initial xylose × 100 [38]
3.7. Biorefinery process
In terms of sustainability, the integrated biorefinery concept is significantly more important than traditional notions, which have drawbacks such as a lack of comprehensive techno-economic assessments and life cycle analyses. An integrated biorefinery process including product processing and extraction, as well as biomass pretreatment and additional processing, results in continuous production. Energy generation followed by waste stream recycling with practically zero discharge. The integrated biorefinery's overall profitability relies on various raw materials and acquired goods as output. To attain the most cost-effective and environmentally favorable alternatives for integrated biorefinery, advancements in raw material selection, conversion procedures, and separation techniques were required. Different types of biorefineries play an important role in raw material usage. The biomass fractionation incurs the greatest capital cost in an integrated system of biomass valorization due to the installation of expensive pretreatment and distillation equipment [39].
The Biofine process, which was patented in 1990, is a modern refining idea for biomass that leads to furfural as an intermediate product in the synthesis of levulinic acid. It consists of two reactors treating various biomass types using acid hydrolysis (1.5-3% dilute sulfuric acid, depending on the feedstock). The initial reactor downstream of a pre-mixer is a plug flow reactor with a short residence period that operates at 210-220 °C. Steam is used to hydrolyze cellobiose at 25 bar. The second reactor is a well-mixed reactor with favorable conditions for Levulinic Acid production, namely 190-200 °C, roughly 20 minutes of residence time, and 14 bar pressure. Concerning the mass yield of furfural, based on the process principle, a value of around 70% of the theoretical yield from C5 sugars derived from biomass is given, which is roughly equal to 50% of the mass [40].
- Effect of Various Parameters on Furfural Production
Effect of different parameters shows the temperature effect, pressure effect, pH effect, and effect of substrate concentration in the furfural production.
4.1. Effect of temperature
Sludge particles were compelled to cram into the holes and gaps of biomass (such as FR and CS) at higher temperatures (such as 100 °C) and pressures of 160 MPa, increasing the contact area of the particles. When the temperature was 100 °C, as opposed to 130 °C, the moisture in the biomass served as a binder. For all sludge mixing ratios, higher particle densities were detected in CSSPs produced at 200 °C than in those produced at DT of 70–130 °C [16]. Temperature has a substantial impact on the furfural yield because it is an essential component of all thermochemical processes. The increase in reaction temperature accelerates the depolymerization of linkages between the carbon bonds of pentosan as well as the xylose dehydration to furfural [10]. The impact of microwave-heated alkali pretreatment on the yield of reducing sugars obtained from lignocellulosic biomass, that as the pretreatment temperature increased from 190 to 230 °C, the yield of reducing sugars decreased to 0.008 g/g TVS (total volatile solids) [41]. This decline in reducing sugar yield can be attributed to the breakdown of reducing sugars into smaller byproducts such as furfural and acetic acid. Therefore, the increasing temperature of pretreatment process resulted in the degradation of the reducing sugars into smaller, less useful byproducts. The normal range is between 220 °C and 240 °C for the production of furfural from pentosan is rice husk [7]. The hydrolysis processes were carried out at various catalyst concentrations (0.06-0.75 M), durations (20-80 min), temperatures (160-200 °C), and feed masses (1.84-3.68 wt.%) [6]. Only 8.52 percent of the furfural was produced at 150 °C, and only 51.4 percent of sugar was converted. Furfural output climbed quickly to 57.01 percent at 160 °C [21].
Based on the amount of C5 sugars in the dried water hyacinth at T=200 °C, the maximum furfural output for water hyacinth was 53.2 mol percent (34 wt. percent) [20]. This is because furfural forms more readily at high temperatures (200-230 °C) [42]. The behavior and reaction of both dissolved and undissolved monomers are influenced by time and temperature, as the majority of the initial materials consist of lignocellulose in nature [43]. By running reactions between 10 and 30 minutes at different temperatures, the impact of reaction time and temperature on the generation of furfural was examined, 8 weight percent pentose sugars at 160 °C to 180 °C, respectively [44]. A lignin-rich residue with less carbon and oxygen than the initial furfural residue is created by Treatment 1 (145 °C, 0.35 M NaOH). Treatments 2 (100 °C, 0.35 M NaOH) and 3 (100 °C, 0.175 M), on the other hand, compared to the initial furfural residue, the hydrogen content of the residue was raised by both (NaOH) [22]. The maximum value of a furfural yield of 49% was achieved at 200 °C, which was the ideal reaction temperature [18]. The sulfuric acid flow is adjusted in accordance with the mass flow from the pretreatment stage to reach an acid concentration of 0.1M under the reaction conditions of 190 °C and 13.14 atm [45]. The generation of furfural is more notable at 150 °C, and the pretreatment process, which is merely water-based, is sufficient to regenerate the catalyst at least once [46].
As it can be seen from the above table, in hydro-distillation process around 170 °C temperature is used. When using the hydrolysis process, 100 °C- 200 °C temperature was applied. While using the acid hydrolysis process, the temperature was raised between 175 °C to 225 °C. In the process of batch type reactor more temperature required and it was 200-300 °C. In the degradation the average temperature was 125-200 °C.
4.2. Effect of pressure
Sludge particles were compelled to cram into the holes and gaps of biomass (such as FR and CS) at higher temperatures (such as 100 °C) and pressures of 160 MPa, increasing the contact area of the particles [16]. The sulfuric acid flow is adjusted in accordance with the mass flow from the pretreatment stage to reach an acid concentration of 0.1M under the reaction conditions of 190 °C and 13.14 atm [45]. Even if the MC was quite high, the moisture was reduced more quickly when the pressure was increased (e.g., 130 MPa and18 percent). At comparatively high MC, increased pressure drove the material to exit the die without sufficient control [16].
4.3. Effect of pH
The pH scale is used to determine how acidic or basic a solution is. As a result, the products' respective pH values when evaluated in a lab were 1.8, 0.5, and 1.23. We deduced from the findings that the product's acidity rises over time. To put it another way, the product's growing acidity is a sign that it contains a lot of furfurals [47].
4.4. Effect of substrate concentration
The maximum mass percent yield of furfural can be synthesized at the best parametric conditions of 199.14 °C, 0.297 M, and 20.27 minutes, which correspond to a 37.90 percent concentration [15]. The samples with the highest hemicellulose content provided the highest furfural yields under moderate conditions (0.1 M MSA), and there was a roughly linear relationship between the two [6].
From the above table, in case of hydrolysis process the substrate concentration was 4.0-5.5 M or 8% v/v or 40:60% v/v. In the degradation process the substrate concentration was 0.1 mol L−1. In case of acid hydrolysis process, the substrate concentration was 1.25% w/w, 1% (w/v), or 300 ml of 1 M or 0.35 M NaOH.
- Development in Furfural Production
Furfural, a biomass-derived polymer, has been widely utilized to demonstrate the synthesis of alternatives to petrochemical-derived polymers. Furfural has been widely employed in the plastics, pharmaceutical, and agrochemical sectors as a natural precursor to various furan-based compounds and solvents. Furfural has been manufactured industrially in mineral-acid solutions (aqueous-acid catalytic solutions). Environmental issues, low yield, and poor furfural selectivity (from hemicelluloses) are the primary downsides of these techniques, which have resulted in the closure of multiple furfural manufacturing plants. Furfural is regarded as one of the most promising value-added compounds that may be manufactured from lignocellulosic biomass due to its wide variety of uses. Biphasic systems have produced more promising findings in terms of furfural yields and selectivity. Because recovering solvents is a difficult operation, these devices may not be commercialized very soon. Globally, large quantities of lignocellulosic residues (wastes) from various agro and forestry-based businesses are accessible. These materials have been investigated as feedstock for the furfural synthesis. In terms of furfural yields and selectivity, biphasic systems have yielded more promising findings. Because recovering solvents is a difficult operation, these devices may not be commercialized very soon [48]
There are several homogeneous catalytic reaction systems with interesting application prospects. However, various obstacles remain in the development of homogeneous systems, including effective recycling of catalyst and solvent, green reaction system building, in-depth knowledge of reaction mechanism, and reactor scaling up. Future study will concentrate on catalysts, solvents, and reaction processes. They are as follow:
- Building a complete reaction network,
- Development and large-scale application of homogeneous catalyst,
- Reasonable selection of solvent system,
- Optimization and enlargement of reaction process, and
- The synergistic development of homogeneous and heterogeneous catalytic reaction systems [49].
Furfural upgrading technology should yet be improved to create cost-competitive biofuels. Further improvements in selectivity and catalyst lifetime, for example, are required. However, the most significant and difficult changes are required in the production of furfural. Furfural may be converted into a number of fuel components. This upgrade seeks to: (i) eliminate the polarity of the aldehyde group to mix in hydrocarbons and (ii) lower the volatility to blend into diesel. Hydrogenation can be improved by combining it with acid-catalyzed rearrangement or etherification, acid-base-catalyzed coupling, or metal-catalyzed decarbonylation. Furfural upgrading can provide either gasoline or diesel mixing components [50].
The urgent need for green and sustainable techniques for converting renewable biomass into commercial chemicals is well acknowledged. Protein engineering, synthetic biology, and metabolic pathway engineering breakthroughs in the last decade have opened the way for the development of efficient enzymatic hydrolysis of lignocellulosic feedstocks and fermentation of the resultant sugars to a range of commodity chemicals [51]. In the near future, study into the fundamentals of the process is also required. Improved kinetics and thermodynamic phase behavior investigations are required to confirm chemical reaction routes and obtain better fundamental understanding about the integration of reaction and product separation to dramatically improve product yields [40].
Industrial furfural yields from lignocellulosic residues digested with sulfuric acid, and then stripped with steam have remained at or below 50% (molar) of theoretical. Brownlee made one of the earliest attempts to enhance furfural yields using a two-step method that first hydrolyzed the pentosans in a hot acid solution before subjecting the wet matter to superheated steam that constantly extracted furfural and eliminated moisture. The rise in hydrogen ion concentration caused by water loss and higher temperatures resulted in a considerable decrease in reaction time. The Quaker Oats Company used a continuous version of this method to achieve 55% yields. Removing furfural from the catalytically active phase as soon as it develops is a more effective technique for increasing furfural yields. The use of solid metal chloride catalysts has showed promise in the search for more ecologically acceptable alternatives to mineral acid catalysts [3].
The use of common acid catalysts (mineral acids such as H2SO4 or HCl) for producing FF creates significant environmental issues. It has several undesirable side effects that result in the era of large quantities of hazardous waste. Therefore, environmentally friendly catalytic processes must be developed to solve both the problem of waste disposal and environmental corrosion. These procedures experience the severe drawbacks of homogeneous catalytic methods, including difficult mineral acid separation and recycling, product contamination, and decreased FF yield due to extended residence periods [52]. Recent advances in the conversion of lignocellulosic biomass, particularly at the commercial scale, are anticipated to fuel the circular and bio-economy. Along with solid catalysts, pyrolysis, gasification, and hydrolysis are common processes that may be used for a variety of feedstocks. However, the behavior of the product can differ based on the composition of the feedstocks. Major barriers to spreading the technology to the burgeoning biofuel and biorefinery industries include economies of scale and competitiveness with petroleum and petrochemical refineries. As the green refinery idea is put into practice, it is necessary to maximize the cost-effectiveness and quality of fractionated products. To uphold the quality of raw materials, a standardized procedure for harvesting, collecting, and storing biomass should be started [53].
- Conclusion
In conclusion, this review study has provided a detailed overview of the manufacture of furfural from lignocellulosic biomass. Furfural, an essential platform chemical with diverse applications has a tremendous promise as a renewable and sustainable alternative to petroleum-based chemicals. Furfural production offers a prospective option for producing a more sustainable and economically successful bio-based economy using lignocellulosic biomass, a plentiful and readily available feedstock. The review focused on the important processes in the manufacturing process, such as biomass pretreatment, hydrolysis, and dehydration, with a specific emphasis on various technologies and methodologies used. Catalyst selection, reaction conditions, and process optimization methodologies were also reviewed, with an emphasis on their impact on furfural production efficiency and selectivity. Mineral acids, solid acid catalysts, and bifunctional catalysts have all been studied for their ability to catalyze the conversion of sugars into furfural. Temperature, residence duration, and feedstock concentration have been further found as important variables impacting furfural synthesis efficiency.
From the above discussion, it can be concluded that rice husk can yield a high percentage of furfural, approximately 90%, when using an acid hydrolysis process at temperatures ranging from 100 to 200 degrees.
Orcids
Md Riajul Islam Sardar: https://orcid.org/0000-0003-0162-6948
Fardaush Hasan: https://orcid.org/0009-0001-8170-8451
Md. Jone Alam: https://orcid.org/0009-0000-7246-2720
Imam Hossain Nadim: https://orcid.org/0009-0007-8431-8786
Md. Mahmud: https://orcid.org/0000-0002-1487-2876
Acknowledgements
We are very thankful to Applied Chemistry and Chemical Engineering Department, Bangabandhu Sheikh Mujibur Rahman Science and Technology University. We are also very thankful to our honorable teacher due to their precious support and encouragement.
Citation: M. R. I. Sardar, F. Ahmed, M. J. Alam, I. H. Nadim, M. Mahmud*. A Brief Review of Recent Furfural Production from Lignocellulosic Biomass: State of the Art of Processes, Technologies, and Optimization. J. Appl. Organomet. Chem., 2023, 3(2), 108-122.


