Document Type : Original Article
Authors
1 Department of Chemistry, Velammal Engineering College, Chennai, India
2 Chemistry of Natural and Microbial Products Department, National Research Center, 12622, Cairo, Egypt
3 Department of Biotech, North East Frontier Technical University, Arunachal Pradesh, India
4 Department of Electronics & Telecommunication, Thakur College of Eng., Mumbai, India
5 Department of Electronics &Communication Engineering, Velammal Engineering College, Chennai-600066, India
Abstract
The consumption of microorganisms proposed to connect metal nanoparticles is in the glow of advertising of modern nanotechnology. Performs as a biodegradable and joyful loom, designed for the assembly of nanoparticles, appreciations to which it is necessary for squat, ecological compatibility, reduced production expenses, the scalability, and stabilization of nanoparticles are compared in bodily and chemical combination. Biologically connected metal nanoparticles are almost all well-organized miniaturized, usable resources constructed and designed to perform precise functions in the company of enormous prospects. Microbes include this amazing competence towards appearance, such delicate nanostructures. This studies the exercise information of organic combination of zinc oxide and lead nitrate nanoparticles as a result of microbes. Microorganisms engage in recreation directly or indirectly in more than a few biological behaviors because metals present in soil are in constant relation to biological components. In the current study, the reported microbiological combination of nanomaterials uses organic ingredients, primarily prokaryotes and eukaryotes, such as bacteria and fungi (Escherichia coli and Aspergillusniger). Bacterial and fungal cell buildup is questioned among two different chemical salts (ZnO and Pb (NO3)2) together with metal nanoparticles should be effectively synthesized.
Graphical Abstract
Keywords
Main Subjects
Introduction
Almost all abundant organisms in our biosphere are bacteria such as Escherichia coli. Invalid climate change can potentially be unfortunate for the bacterial process; they know how to do this during the inexhaustible benefits of nanoparticles [1]. Alternatively, a combination of metal nanoparticles as a result of eukaryotic cells, such as observing that Aspergillusniger fungi are reported [2]. A. niger includes the benefit of producing unbelievably far above the ground of veiled protein crops, which is likely to increase the rate of nanoparticle connection [3]. Mycelia provides a much privileged external neighborhood than bacteria, and this area is likely to be second-hand to maintain the interface of metal ions and the falling fungus negotiator, which is a pleasant eye drop in metal nanoparticles [4].
At the same time, when physical and chemical methods appear to connect nanoparticles to maintain expensive and inexpensive, scientists are developing cheaper processes for the nanoparticles expansion using microorganisms, which can be obtained in an environment [5]. The nanomaterials biosynthesis persists for the most part in an unknown neighborhood, which requires in the context of depth research [6]. Motivating to talk about this environment is a pool of numerous microorganisms and constructive phytochemicals for the further existence of the entire cosmos and protection of humanity [7]. The natural world has the inherent competence to formulate processes designed to combine nano- and micro-scaled inorganic materials [8]. This opened an innovative panorama of research preceding science and technology. Nanoparticle biosynthesis generally includes oxidation or reduction development similar to different bottom-up approaches [9]. During this progress, microbial enzymes/plant phytochemicals are involved in recreation as part of reducing metal compounds within their individual nanoparticles [10].
Microbes or phytochemicals that antioxidant or reduce potential are reliable as part of this progress, in line with the path to a thorough study. Nanoparticle biosynthesis is to implement the standard of green chemistry. The most important stages related to progress are [11]: The solvent medium used for the combination is preferred, an alternative, for environmentally benign as reducing agent. The preference nonhazardous bits and pieces for stabilize nanoparticles, since the bio organism is involved in the biosynthesis process, the biosynthetic tube for the nanoparticles production is in line with the doctrine of green chemistry, because the bio-organism used as a reducing agent, and the restrictive measure is recyclable [12].
Promoting progress is that the chemical combination includes the introduction of chemical compounds into the game that may be harmful to the environment, relax on the facade, making adverse special effects for people in the circumstances of infection, infection verdict, and health control management. This category of circumstances does not occur in a biosynthetic alley for the nanoparticles synthesis due to its biodegradable temperament and bio-concompetitive effectiveness in medicinal and pharmaceutical applications [13]. Thus, the biosynthetic pathway is beneficial compared to chemical methods for the nanomaterials production. By nature, biosynthesized environmentally friendly nanoparticles do not cause any negative effects/reactions in medical applications. The green synthesis of metal nanoparticles as such silver, zinc, and lead was agreed using CorriandrumSativum leaf extract for the ecological development of new technologies. We have developed an easy and environmentally friendly method of synthesizing zinc oxide nanoparticles using CorriandrumSativum water leaf extract with zinc acetate dihydrate as a precursor.
ZnO is widely used in water purification. ZnO nanoparticles are used to remove arsenic and sulfur from water, although mass zinc oxide cannot absorb arsenic. This is due to the fact that nanoparticles have much larger surface areas than mass particles. Phytochemical plant material with antioxidant properties is responsible for the preparation of metal nanoparticles and metal oxide. Recently, the nanoparticles synthesis with bacteria, fungi, actinomycetes, and the use of plant extract such as neem, cameliasinensis, Corriandrum, nelumbolicifera, ocimumsanctum, and several others has been obtained, which comply with the principles of green chemistry.
Microbial synthesis
The microbiological synthesis of nanoparticles involves the use of microorganisms. This approach is environmentally friendly and is widely used for the nanoparticles synthesis. Biological components such as prokaryotes and eukaryotes are the main source of microbiological synthesis of nanoparticles. Several important biological processes in nature are regulated by microorganisms. There is a lot of evidence that there is a permanent relationship between biological components and metals, as well as non-metals existing on earth [12]. It is mentioned that bacteria are abundantly present in the biosphere, and any small climate change can adversely affect their life processes, thus affecting the synthesis of nanoparticle microorganisms. Microbial cells used to produce large nano-materials have opened an ecological approach to the production of metal nanoparticles.
Although efforts to bio-fine nano-materials are new, the interaction between metals and microorganisms has been well documented, and the ability of microorganisms to accumulate or extract metals is used in biotechnological commercial processes, such as bioremediation and bioleaching. It is known that fungi and bacteria synthesize inorganic metal nanoparticles intra cellular/extracellular cells.
Bacteria
The studies focused mainly on prokaryotes (E.coli) as a method of synthesis of metallic nanoparticles. E.coli is presented in abundance in our biosphere and can adapt to extreme environmental conditions or survive in adverse environmental conditions. Therefore, bacteria have been recognized as the best candidate for microbiological synthesis of metal nanoparticles and related studies. Bacteria grow faster even in extreme environmental conditions. Bacteria can be easily manipulated and grown in our environment. Various biological agents can be controlled, such as temperature, oxygenation, and medium and incubation time necessary for bacterial growth conditions.
It has been observed that the change in pH of the growth medium during incubation facilitates the biosynthesis of nanoparticles and nanoparticles of various sizes and shapes [13]. Given that nanoparticles have opened up a broader meaning in industry, the environment and health, suitable nanoparticles of incredible size are necessary to accumulate a provision. This required an appropriate assessment of the intensification environment for control, seeing that more than bacteria intended for the production of nano-materials in experimental/laboratory conditions were mentioned. Some examples of bacteria used to make inorganic metal nanoparticles are pre-arranged below: magnetotactic bacteria are used to connect magnetic nanoparticles. S-layer bacteria are used to form gypsum and calcium carbonate layers.
Furthermore, studies have shown that a number of microorganisms may still exist to increase the mindfulness of metal ions because they include the metal confrontation function [14]. It can be predicted that the mechanisms of microbial metal confrontation include an assortment of organic processes along with bio-sorption, modification of toxicity and solubility from beginning to end with oxidation or reduction, discharge systems, bioaccumulation, precipitation or extracellular metal complexation, as well as the lack of clear nanomaterial transfer systems [15]. One case of metal confrontation with the action of microorganisms is Pseudomonas stutzeri AG 259 [6]. This micro-organism can grow and 5 even at high metal ion concentrations in silver mines that have the ability to produce silver nanoparticles [16].
An additional case here is also recommended for the production of gold nanoparticles via bacteria. It is a replacement method for joining gold nanoparticles [17]. Rhodo-pseudomonas capsulata have bacterium for the connection of extracellular gold nanoparticles of specific sizes 10-20 nm and recommended that these nanoparticles be formed using the NADH-dependent reductase enzyme. NADH-dependent reductase is an enzyme published earlier as necessary for metal biosynthesis [18].
Fungi
The micro-organism, ‘fungi’, is also an additional biological type for the production of metal nanoparticles [19]. The fungus has the ability to biosynthesis of the metal oxide nanoparticle assortment [20]. Mechanisms for the formation of nanoparticles using fungi have been planned for incredibly few nanoparticles and recognized in the technical literature [21]. Mushrooms recognized the noteworthy awareness of the production of metallic nanoparticles more than bacteria due to information that fungi convinced to compensate in the bacteria assessment [22]. Without effort to increase scale and deal down, monetary profitability and being there in my style, greater than in front of the facade, they are necessary compensation for meditation.
Because Aspergillus niger mushrooms gush with significantly increased amounts of proteins than bacteria, this would increase the efficiency of nanoparticle connection [23]. Although fungi are able to be used to connect nanoparticles with a consistency in their dimension and silhouette, fungi may also be required to produce nanoparticles with different proportions [24]. Mushrooms include the ability to leak further amounts of (proteins, which causes glycosylated) proteins to produce a huge number of [25] nanoparticles. Therefore, fungi can be a rich starting point for the production of nanoparticles in large quantities in the evaluation of bacteria [26]. Almost none of their examples, such as Fusarium oxysporum and Aspergillus niger, were mostly purposeful by major scientists by observing how to call inorganic metal nanoparticles [27]. Numerous mechanisms have been completed especially towards their silver nano-materials plant [28]. Antiseptic silver nanoparticles (AgNP) were synthesized in the size range of 5-15 nm, and it was possible, that they were limited with the intention of their maintenance from the beginning to the end of protein secretion by the fungus [29]. Although almost everyone necessarily goes forward in the fungal combination of natural nanomaterial, because A. niger and F. oxysporum will produce these extracellular nanoparticles, unlike any previous study, in which only intracellular production of Ag and Au NP [30] .
Experimental
Materials and methods
Preparation of LB broth (1.5%)
To organize (1.5%) of LB broth medium, 1.5 gm LB broth medium was weighed and dissolved in 100 ml distilled water, autoclaved, and stored at room temperature (RT). Supplementary chemicals in employment in this revision are PDB, 1 M NaOH and HNO3, (Pb (NO3)2), and (ZnO) were procured from Sigma. The utilized microbes were Escherichia coli (DH5a) (bacteria) and Aspergillus niger (fungus).
Preparation of bacterial growth medium for the synthesis of nanoparticles (E.coli)
Intended for the foreword of augmentation intermediate, the subsequent procedure is used:
The bacterial strain E.coli (DH5a) second-hand in the experimentation was purchase from Pks, Infra Engineers Pvt Ltd, Ghaziabad. Broth formulations like LB (Luria Bertani) intermediate which contain two dissimilar changeover metal salts (Pb(NO3)2 and ZnO) was equipped by means of the preferred concentration.
LB media preparation-(for 1000 ml solution)
Peptone 5 gm
Beaf extract 3 gm
NaCl 5 gm
PH 7.2
LB media suitable for bacterial culture
Chemical salts:
Pb (NO3)2 331.21 g/mol
ZnO 81.38 g/mol
Procedure of Nanoparticle synthesis using bacterial strain E.coli (DH5a)
Intended for speedy growth of the bacterial cells, LB medium was organized and uncontaminated for each experiment. The obtained bacterial strain of Escherichia coli (DH5a) was cultured in LB medium to bring into being the biomass for biosynthesis [31]. Inoculation of bacterial ethnicity was conceded out in Luria Bertani (LB) intermediate using the shake flask technique [32]. 1.5% of LB broth was geared up in Erlenmeyer flask (250 ml) by mixing of 1.5 g of LB broth powder in 100 ml distilled water, and then, it was autoclaved and the intermediate was permissible to cool up to 40-45 ℃.
Flamed the neckline of the flask, chosen a single colony by sterile loop from culture plate, and put that loop in to the flask. The flask was labeled to point toward date and strain. On one occasion, inoculated the flasks were incubate during the night in an incubator shaker at 250 rpm speed, at 37 ℃. Three sets of 25 ml test tube were also uncontaminated previous to carrying out tests. 10 ml of LB medium was transfer to all test tubes. An assortment of concentrations of (1.5 mM) chemical salt of ZnO and Pb(NO3)2 were cautiously positioned into all test tube, leave-taking one as a be in charge of to track the standard enlargement of the microbial cells devoid of salt after that autoclaved it. It was cooled at (45-50 ℃) at normal room temperature in laminar air chamber. A piece of test tube was then inoculated with 50 ul of E.coli (DH5a) grown in liquid LB medium. Apiece test tube was shaken using a rotary shaker at 180 rpm at 37 °C for 72 h (Figure 1).

Figure 1. The nanoparticle synthesis using bacterial strain E.coli (DH5a) (a) synthesis of ZnO nanoparticles and (b) synthesis of Pb(NO3)2 nanoparticles
Preparation of fugal growth medium for the synthesis of nanoparticles (Aspergillus niger)
The fungus Aspergillus niger used in the experiment was purchased from Pks, Infra Engineers Pvt Ltd, Ghaziabad. Potato dextrose broth/PDB which contains two different transition metal salts (Pb (NO3)2 and ZnO was equipped with two different (low and high) concentrations.
PDB media preparation-(for 1000 ml solution)
Potato 200 gm
Dextrose 20 gm
PH 5.6
PDB is suitable for fungus culture
Chemical salts
Pb (NO3)2 331.21 g/mol
ZnO 81.38 g/mol
Procedure of Nanoparticle synthesis using fungal extract Aspergillus niger
Biomass of fungus A.niger (purchased) was cultivated in potato dextrose broth (PDB), using the shake flask method [33], as described in section 3.4.1 with deference of PDB. From beginning to end the make use of loop, fungus culture was transferred into an Erlenmeyer flask (250 ml) containing 50 ml PDB [34]. Subsequent to inoculation, flasks are stunned at 150 rpm speed on a rotary shaker at 30±2 ℃ for 3 days. The culture grew like discrete pellicles. Biomass harvesting was carried out through filtering, and then washing the biomass, known as “viable biomass”. The viable biomass of pellet was utilized in the salt uptake studies. The salt tolerance of mycelial growth was investigated through weighing the dry biomass of pellet after 3 days incubation at 150 rpm speed on a rotary shaker at 30±2 ℃ in the potato dextrose broth with desired concentration of chemical salts. Weighted dry biomass of fungus in 100 ml distill water + Pb (NO3)2 and ZnO at the concentration range of 10 mg, as compared with that was without salt used as control. Usually, 10 g of wet weighted biomass were introduced into the contact of sterile doubled distilled water of 100 ml in an Erlenmeyer flask at 27 C for 48 hours that agitated at 150 rpm. After incubation, Whatman filter paper no. 1 was used to filter the cell filtrate. Once filtered, the pH of observed cell filtrate was adjusted at 7.2, using 1 M NaOH and HNO3. In to the 50 ml of cell filtrate, a carefully weighed quantity of chemical salt, ZnO and Pb (NO3)2 was added to the Erlenmeyer flask, (25 ml filtrate + Pb (NO3)2 in one flask and 25 ml filtrate + ZnO in another) and incubated at normal room temperature in dark. Control, use in this protocol, containing cell free filtrate without any salt concentration was run simultaneously as standard with the experimental flask. According to Pimpa W. et al. (2004), nanoparticles were concentrated through shaken at 125 rpm at 30±2 ℃ on a rotary shaker for 2 hours (Figure 2).

Figure 2. Synthesized of nanoparticle using fungal extract Aspergillus niger (a) synthesis of ZnO nanoparticles and (b) synthesis of Pb(NO3)2 nanoparticles
Instrumentation
Electron Microscopes (TEM and SEM) was used to confirm the formation of ZnO and Pb (NO3)2 nanoparticles from the microbes. Incubator, Autoclave, Rotary shaker Laminar air flow, and Nichrome inoculating loop.
Selection of microbes to synthesize metal oxide nanoparticles are Escherichia coli (bacteria) and Aspergillus niger (fungus). Heavy metals at relatively low concentration are considerably hard to remove through conventional techniques [35]. A feasible alternative to realize this kind of removal can be through the bio-sorption application by bacterial and fungal biomass. This research was carried out to estimate the removal of lead nitrate and zinc oxide from an aqueous solution by biomass of Escherichia coli and Aspergillus niger [36]. Essential chemical salts used in the protocol are lead nitrate (Pb (NO3)2) and zinc oxide (ZnO) [37]. Lead and zinc are a heavy metal, sometimes may be poisonous (regardless of it swallowed or inhaled), affecting almost every system and organ in the body [38]. Nervous system is the major target for the lead toxicity. Lead based poisoning generally results through ingestion of water or food that contaminated with Pb; it may also exist after accidentally ingestion of contaminated lead-based paint, dust, or soil. Bioremediation of zinc and lead through microbes could be a functional measure to remove zinc and lead [39-43].
Results and Discussion
The use of microorganisms intended for the production of nanostructured resources appears to be exciting in addition to the environmental approaches. Scientists usually prefer organic bonding because particle allocation is responsible for how to stay from beginning to end, this technique is better than further methods. In addition, organic technique is also not associated with organic toxicity so that it is usually accompanied by a further chemical method. The microbiological combination of nanostructured particles means that primarily organic resources of prokaryotes and/or eukaryotes are involved. Microbes achieve direct/indirect roles in various organic activities. The range of physical and chemical methods worn to produce nanoparticles has been sick since the start of more than a few restrictions. They include areas of this technique; they are most often biocompatible, as well as venomous, unfettered, unfettered crystal growth and aggregation of nanosensated particles. Towards the conquest of these districts in the modern decade, contemplation towards the nanoparticles construction using biological components is aware in advance, just as biological methods include environmentally friendly, harmless, and hygienic means of production and collection of nano-materials. The biological technology procedures almost resourceful nature equipment that is living cells to produce.
Study of nanoparticles synthesized from bacterial strain E.coli (DH5a)
The revolutionized color obtained in the convenience of tubes containing chemical salts gave a prelude to the combination of nanoparticles. The resulting filtrate/biomass was alienated, starting each tube from centrifugation from beginning to end, with the intention of admitting to exposure at 7,000 rpm/min and unlocking the supernatant for an auxiliary representation. Fashionable nanoparticles are characterized by a UV-Vis spectrophotometer, wherever visual compact measurements starting in each tube have been prepared to monitor the reduction of synthesized nanoparticles and electron microscopes (TEM and SEM) to confirm the combination of ZnO and Pb(NO3)2 nanoparticles (Figures 3 and Figures 4).
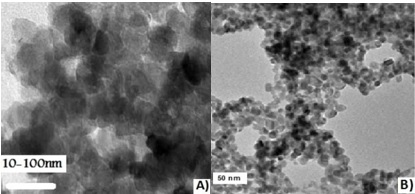
Figure 3. TEM micrograph of (a) ZnO and (b) Pb(NO3)2 nanoparticles

Figure 4. SEM micrograph of (a) ZnO and (b) Pb(NO3)2 nanoparticles
Study of nanoparticles synthesized from fungal extract Aspergillus niger
The color change obtained in the content of flasks containing chemical salts gave an initial signal of the connection of nanoparticles combination. The resulting biomass was alienated, starting each flask from spin from beginning to end, which disappeared at 7000 rpm and composed a supernatant to promote categorization. Shaped nanoparticles were characterized by a UV-Vis spectrophotometer everywhere there, where the dimensions of the eye compactness starting with the carafe were filled to monitor the decrease of nanoparticles every 24 hours and the documentation of fungus intensification (Figures 5, 6, 7, and 8).
The description of metal ions (lead nitrate and zinc oxide) opens to the elements to microbial strain and the lessening of these metal ions to individual nano-materials were long-established by the UV-Vis spectrophotometer. Subsequent to the adding together of lead nitrate and zinc oxide to the bacterial culture, the synthesize nanoparticle elucidation was scanned from beginning to end the UV-Visible spectrophotometer. ZnO NPs have pointed absorbance in the midst of the utmost crest at 300 nm and Pb(NO3)2 NPs have razor-sharp absorbance in the midst of the uppermost hit the highest point at 250 nm, correspondingly. In the same way, subsequent to the adding together of zinc oxide and lead nitrate to the fungal culture, the synthesized nanoparticle elucidation was scanned UV-Visible spectrophotometer. ZnO NPs have razor-sharp absorbance in the midst of the uppermost peak at 230 nm and Pb(NO3)2NPs have prickly absorbance in the midst of the uppermost peak at 240 nm, correspondingly. The distinguishing of metal oxide nanoparticles was more evidently pragmatic in the supernatant solutions of fungal strains representative the NPs amalgamation. It was pragmatic that a superior lessening of metal oxide nanoparticles in the growth medium of fungus A. niger subsequently bacteria E. coli for the duration of the 24-hour incubation, reduce in the radiation concentration in a straight line comparative to the attentiveness of the solution. A. niger have the benefit of produce extremely elevated yields of concealed proteins as well as an augmented surface area, which possibly will increase nanoparticle amalgamation rate. Consequently compared to bacteria, A. niger encompasses considerably and boosts the biosynthetic efficiency come up to of metal oxide nanoparticles.

Figure 5. UV-Vis spectrum for the of Pb(NO3)2 NPs synthesized by fungus (A.Niger)

Figure 6. UV-Vis spectrum for the ZnO nanoparticle synthesized by fungus (A.Niger)
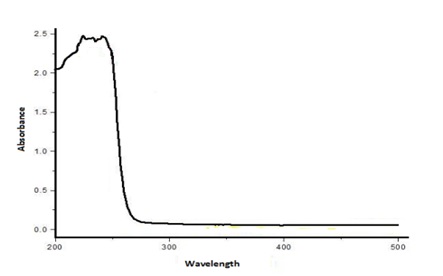
Figure 7. UV-Vis spectrum for thes ynthesis of Pb(NO3)2 NPs through bacteria E.coli

Figure 8. UV-Vis spectrum for the synthesis of ZnO nanoparticles using E.coli
Conclusion
The summary of the excitation from the use of the micro-organism, such as describing bacteria and fungi for metal NP biosynthesis, through methods of shaking the flask currently in fashion. The production of metal oxide nanoparticles from primary to final bacteria (E.coli) in addition to the fungus (A. niger) is highly likely compared to the conventional joining methods. The organic combination of nano-materials, specialist knowledge should be increased to check the effectiveness of the starting price. Connection progress is biodegradable, fast, and follow organic within range of the mechanism. E. coli bacteria are well above ground growth and are relatively despicable to promote in the assessment of other biological systems. Bacteria include a small number of compensation for fungi, because they can be manipulated without difficulty, constructed as an alternative to close bio-production of nanoparticle expression. On the other hand, the A. niger mushroom has the advantage of producing very high yields of unknown proteins, which can increase the rate of nanoparticle synthesis. The fungi of microorganisms include mycelium, which provides an increased surface for a variety of fungi in bacterial evaluation. Therefore, this increased area can be helpful in supporting the fungal interaction reducing agent with metal ions and improving the conversion of ions into metal oxide nanoparticles. Mushrooms also benefit from trouble-free dispensation when nanoparticles are created, allowing a more well-organized way of extracting nanoparticles from them. Scalability, an additional aspect of the council in terms of cost-effective nanoparticle production, gives the fungi a circuit as an alternative framework in the order of long-term expansion, because they can be easily used in large reactors than bacteria. Due to the fact that the A. niger fungus has the ability to secrete a large amount of proteins than E. coli; in this way, noticeably pleasing to the eye, biosynthesis productivity is within the range of production and categorization of metal oxide nanoparticles. The district of the total production of metal oxide nanoparticles is relatively a work of fiction and under-explored. On the other hand, it shows a huge perspective in the biotechnology segment. Presence will be a large aspect of this biological method that will be revealed, and then manipulated, seeing that specialist knowledge will appear. The use of microorganisms for the production of metal oxide nanoparticles is reliable and accompanied by an ecological label.
Orcids
Mohamed Sikkander: https://orcid.org/0000-0002-8458-7448
Fatma Bassyouni: https://orcid.org/0000-0001-6817-1218
Khadeeja Yasmeen: https://orcid.org/0009-0005-2970-011X
Sangeeta R Mishra: https://orcid.org/0000-0002-0123-2650
V.Vidya Lakshmi: https://orcid.org/0009-0009-1328-0594
Acknowledgements
I would like to acknowledge and give my warmest thanks to Dr. P. Manisankar, Former Vice Chancellor, Bharathidasan University, Trichy, and Tamil Nadu, India who made this work possible. I would also like to thank coauthors for letting my defense be an enjoyable moment. Finally, I would like to thank God, for letting me through all the difficulties.
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Citation: A.M. Sikkander*, F. Bassyouni, K. Yasmeen, S.R. Mishra, V.V. Lakshmi. Synthesis of Zinc Oxide and Lead Nitrate Nanoparticles and their Applications: Comparative Studies of Bacterial and Fungal (E. coli, A. Niger). J. Appl. Organomet. Chem., 2023, 3(4), 255-267.


