Document Type : Original Article
Authors
- Saba Ibrar 1, 2
- Naveed Zafar Ali 1
- Ernest O. Ojegu 3
- Ogo B. Odia 4
- Imosobomeh L. Ikhioya 1, 5
- Ishaq Ahmad 1, 6
1 National Centre for Physics, Quaid-i-Azam University Campus, Islamabad, 44000, Pakistan
2 Department of Physics, Quaid-i-Azam University, Islamabad 45320, Pakistan
3 Department of Physics, Delta State University, Abraka, Delta State Nigeria
4 Department of Physics, Delta State College of Education, Mosogar. Delta State, Nigeria
5 Department of Physics and Astronomy, University of Nigeria Nsukka, 410001, Nigeria
6 NPU-NCP Joint International Research Centre on Advanced Nanomaterials and Defects Engineering, Northwestern Polytechnical University, 710072, China
Abstract
Zeolitic imidazolate frameworks (ZIF-8 and ZIF-67) were synthesized for energy storage by utilizing cobalt (II) nitrate hexahydrate, zinc nitrate hexahydrate, and 2-methyl imidazole. The redox peaks in the CV plots showed the existence of faradaic processes. The specific capacitance of ZIF-8 and ZIF-67 reduces with an increase in scan rate. ZIF-8 and ZIF-67 have estimated specific capacitances of 625, 312, 208, 156, and 125 F/g and 932, 468, 312, 234, and 187 F/g at scan rates of 10 to 50 mV/s. ZIF-8 and ZIF-67 have electrolyte resistances of 1.25 and 1.15 Ω, respectively, with active electrode resistances of 0.03 and 0.02 Ω. Great capacitive performance was indicated by the low charge transfer resistance seen in the Nyquist plots. Both ZIF-8 and ZIF-67 have estimated specific capacitances of 276.91 F/g according to the GCD tests. ZIF-8 and ZIF-67 had an energy density of 7999.89 when the current density was 2.0 A/g. ZIF-8 and ZIF-67 nanocrystals differ in their XRD patterns, as the structure of ZIF-8 remains intact. The nanoparticles exhibit high crystallinity, as evidenced by the distinct peaks observed, particularly the sharp (001) peak at 7.4o. The high-pitched and defined peak at the 2θ position of 7.6° confirmed the crystalline nature of synthesized ZIF-67. The (011) crystal plane was identified as the source of the intense peak at 2θ position 7.6°. The bandgap value of ZIF-67 is 3.27 eV, in contrast to ZIF-8 which has a value of 3.0 eV.
Graphical Abstract
Keywords
Main Subjects
Introduction
The significance of energy storage devices is higher than ever. These devices will play a crucial role in future energy technologies [1]. They will provide power for transportation and store energy from intermittent sources like solar and wind power. Supercapacitors, much like batteries, have gained recognition lately for their eminent power generation capabilities [2]. In contrast to batteries, supercapacitors have a limitation that they cannot hold as much charge [3]. In spite of their superior power delivery capacity, they can typically only hold around 3 to 30 times less charge than batteries. Supercapacitors can improve energy storage systems based on batteries by changing their power and energy capabilities, increasing capacity, meeting specific power and energy requirements, and also prolonging their life [4,5]. Future energy technologies will require efficient energy storage devices to meet the demands of electric vehicles, smart grids, and hybrid electric vehicles [6]. Various energy storage devices require improvements in energy density, cycle, and rate performance [7]. Carbon-based supercapacitors are gaining attention due to their long-term durability, high power density, and ability to charge and discharge rapidly, resulting in significant interest. However, their specific capacitance, which quantifies the charge storage per unit mass, stays comparatively low. The performance of these supercapacitors depends largely on the properties of the materials used for the electrodes, including their chemical and physical characteristics [8,9]. Porous carbons are highly desirable in advancing various technologies for a sustainable future. Scientists have shown significant interest in applying metal-organic frameworks (MOFs) as precursors and templates for carbon materials [10]. To develop and shape the porous structure of carbons, MOFs have been utilized through both direct and indirect methods.
MOFs frameworks are gaining more attention as a promising material for energy storage [11]. These materials are typically microporous (<2 nm) and maintain long-lasting porosity, making them useful for various practical applications. MOFs possess greater coordination bond energy (usually 60-350 kJ mol-1) as compared to weaker bonds such as hydrogen and van der Waals bonds. This provides them stability and allows them to create permanent pores [12-14]. MOFs' physical and chemical features, along with their structure, are highly customizable. The number of MOFs has been increasing continuously since the term was coined and now exceeds 20,000, according to researchers. These different center-metal ions can join with diverse organic ligands to create a broad spectrum of MOF compounds [15,16]. MOFs are being extensively studied due to their ability to be used as a highly adaptable substrate in various research fields, owing to their exceptional surface area, vast porosity, variable pore sizes, and flexible functionality. MOFs are one of the best options for electrode materials in lithium-ion batteries due to their precise structural control and high porosity. Zeolitic imidazolate (ZIFs) frameworks, a type of MOF comprised of tetrahedrally coordinated metal ions linked by anionic imidazolate ligands, are well-suited for use as sacrificial templates due to their controllable size and shape, as well as their chemical and thermal stability [17-19]. One significant difference between ZIFs and zeolites is that systematically altering the linker substituents can generate various framework topologies [20,21]. The flexibility in functionalization permits the logical surface property design.
MOFs are well-known materials utilized in different fields like separation technology, catalysis, electronics, sensors, and smart devices. These materials are highlighted due to their extensive surface area, morphology, porosity, and structure. MOFs subclasses vary depending on the metal cation and organic ligand [22-24]. Various applications make extensive use of the soft template MOF ZIF-67. The characteristics of ZIF-67, such as thermal, chemical stability, high catalytic activity, and tunable pore size, make it an appealing option for many research areas and large-scale applications. The combination of ZIF-67 with other constituents or structures may enhance its performance to exceed that of pure ZIF-67. The class of materials known as metal oxide nanoparticles/ZIF-67 has unique functional properties and is becoming more prominent. The metal oxide crystalline structure's functionality is merged with ZIF-67's porosity [25,26]. The performance of ZIF-67/metal oxides has been assessed in various applications, such as adsorption, catalysis, sensing, storage, and microwave absorption.
In the literature, there are various 3D MOFs. However, one of the most popular is Zinc-imidazole framework 8 (ZIF-8), also called Zeolitic imidazole frameworks (ZIFs), due to its unique physicochemical and structural properties [27-29]. The metal part of the ZIF structure can be converted into metal oxide via oxidative pyrolysis or separated into a carbonaceous substance using anaerobic thermolysis. Metal ions or metal oxides are the primary inorganic units comprising redox active sites during the electrochemical process. Lithium-ion batteries (LIB) may use ZIFs as an electrode material. Through pyrolysis, ZIF-8 can be converted into porous carbon and zinc oxide materials with a large specific surface area and high conductivity under both oxidative and anaerobic conditions. ZIF-8 is a great option for producing and adjusting LIBs, as a result. Micro/mesoporous carbons obtained by carbonizing ZIFs have excellent conductivity and a naturally large surface area, making them highly versatile for utilization in lithium-sulfur batteries (LSBs) [3].
Our research involves the synthesis of ZIF-8 and ZIF-67, which are materials we believe will be useful for energy storage applications.
Experimental
Materials and methods
Analytical grade chemicals were employed to synthesize ZIF-8 and ZIF-67, which were purchased from Sigma Aldrich: Cobalt (II) nitrate hexahydrate, Co(NO3)2·6H2O 99.9%, zinc nitrate hexahydrate (Zn(NO3)2·6H2O) 99%, 2-methyl imidazole, C4H6N2 99%), acetone, methanol, and deionized water.
ZIF-8 preparation
The process of making ZIF-8 involves dissolving 2.4 g of zinc nitrate hexahydrate in 30 mL of methanol. A similar procedure was used to dissolve 2.5 g of 2-methyl imidazole in 30 mL of methanol. The aforementioned two solutions were subjected to sonication for the next 30 minutes. The solutions were combined and sonicated for 40 minutes. The solution remained untouched for a whole day. The result underwent centrifugation, was washed with methanol multiple times, and dried for three hours at 800 C (Figure 1).

Figure 1. Synthesis procedure of ZIF-8
Synthesis process of ZIF-67
The ZIF-67 formation involved dissolving 2.4 g of cobalt nitrate hexahydrate in 30 mL of methanol. A solution of 2.5 g of 2-methyl imidazole in 30 mL of methanol was prepared. The two solutions mentioned above were sonicated for 30 minutes. The cobalt nitrate solution turns purple upon the addition of 2-methyl imidazole, and then it is sonicated for 40 minutes to achieve a homogeneous composition. The solution persisted after a day. Next, the final product was centrifuged, washed multiple times with methanol, and dried for three hours at 800 C (Figure 2).
Figure 2. Synthesis procedure of ZIF-67
Characterization of ZIF-8 and ZIF-67
The crystal structures and orientations of ZIF-8 and ZIF-67 were examined using an X-ray diffractometer. Surface micrographs and elemental compositions were examined using scanning electron microscopy. FTIR was used to examine the functional groups present in ZIF-8 and ZIF-67 materials. The optical characteristics of these materials were studied between 200 and 800 nm wavelengths using UV-Visible spectroscopy. Electrochemical studies were conducted using Gamry Potentiostat and Galvanostat.
Results and Discussion
X-ray diffraction (XRD)
Figure 3 displays X-ray diffraction (XRD) patterns for ZIF-8 and ZIF-67 nanocrystals. The XRD patterns of ZIF-8 and ZIF-67 nanocrystals differ, as the structure ZIF-8 remains intact. The peaks are consistent with earlier research, suggesting the formation of ZIF-8 nanocrystals [30]. The nanoparticles exhibit clear crystallinity, as evidenced by the distinct peaks observed, particularly a sharp (001) peak at 7.4o. A body-centered cubic crystal lattice is displayed by the crystals [30]. The crystalline integrity of ZIF-8 materials was preserved, without any structural modifications [31]. Figure 3 demonstrates that the diffraction peaks of the synthesized ZIF-67 matched with the simulated pattern in the literature [32]. The results suggest that the ZIF-67's crystalline structure is well-preserved and impurities are absent. Synthesized ZIF-67 was confirmed as crystalline due to a distinct and intense peak at the 2θ position of 7.6°. The intense peak with a 2θ angle of 7.6° was observed, attributed to the (011) crystal plane.

Figure 3. XRD of ZIF-8 and ZIF-67
UV-Visible spectroscopy
As illustrated in Figure 4 (A1), ZIF-67 showed an absorption edge at about 235 nm, and ZIF-8 showed one at about 260 nm. This suggests that ZIF-8 and ZIF-67 absorb light at slightly different wavelengths. Due to their different crystal structures and metal-ligand coordination environments, ZIF-8 and ZIF-67 exhibit different absorption edges from one another. ZIF-67 has an apparent larger energy bandgap than ZIF-8, as evidenced by the observed blue shift in the absorption edge of ZIF-67. It is obvious from Figure 4 (A4) that ZIF-67 has a bandgap value of 3.27 eV, while ZIF-8 has a bandgap value of 3.0 eV [33]. Across the measured wavelength range, the ZIF-8 UV-visible transmittance curve showed lower transmission levels than the ZIF-67 transmittance curve (Figure 4 (A2). This indicates that ZIF-67 exhibits higher light transmission capabilities than ZIF-67, which allows less light to pass through. Their different molecular structures, porosity, or surface characteristics may be the cause of the observed transmittance difference between ZIF-8 and ZIF-67. The lower transmittance of ZIF-8 might be an indication of a greater level of light absorption or scattering within the substance, which would result in lower light transmission. However, ZIF-67's higher transmittance might suggest that it has a more transparent or advantageous structure. Furthermore, over the measured wavelength range, the UV-Visible reflectance curve of ZIF-8 displayed higher reflectance levels compared to the reflectance curve of ZIF-67, as can be seen in Figure 4 (A3).
 Figure 4. UV-Visible plot of ZIF-8 and ZIF-67
Figure 4. UV-Visible plot of ZIF-8 and ZIF-67
Scanning electron microscopy
The image of ZIF-67 and ZIF-8 showed a defined cube shape with smooth surfaces and sharp edges. The particles possess a uniform size and maintain a consistent cubic structure in the sample. ZIF-67 has a cubic shape with some variations in edge sharpness, just like some things have slight differences. The ZIF-67 particles have a smoother surface than ZIF-8. The size of particles is crucial for the function and effectiveness of these materials. ZIF-8 particles measure approximately 252 nm. Due to its nanometer size, ZIF-8 is ideal for applications such as catalysis, gas separation, photovoltaics, and energy storage. The ZIF-67 particles were measured to be approximately 292 nm in size. The size is not as precise as ZIF-8, which could impact its applicability in certain fields. The tiny and even size of ZIF-8 could boost its effectiveness in photovoltaic and energy storage applications. The larger particle size of ZIF-67, though still in the nanometer range, might be advantageous for applications requiring slower diffusion or controlled release behavior. The well-defined and uniform cubic morphology may facilitate reproducible synthesis and scalability, essential for industrial production (Figure 5).
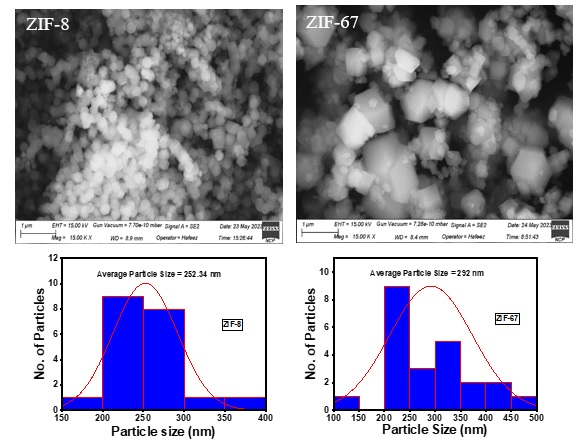
Figure 5. SEM of ZIF-8 and ZIF-67
Fourier transform infrared spectroscopy
Figure 6 (S1) displays the FTIR results of ZIF-8 to identify the functional groups of the materials produced. The prepared sample ZIF-8 exhibited the bands at 3135, 2933, 1586, 1425, 1320, 1152, 999, 761, and 698 cm-1. These bands agree with the previously published data. The band at 3455 cm-1 is due to the oxygen-hydrogen (O-H) stretching vibrations of water following KBr deliquescence and the corresponding N-H stretching vibrations of the remaining Hmim. The aromatic and aliphatic C-H asymmetrical stretching vibrations were linked to the peak at 3135 and 2933 cm-1, respectively. The 1586 cm-1 band was attributed to the C=N stretching vibration, whereas the 1635 cm-1 band was caused by the C=C stretch mode. The band at 1146 cm-1 represented the C-N stretching mode of the aromatic compound, while the signals between 1300 and 1460 cm-1 indicated the stretching of the entire ring. The vibration of the C-N and C-H bending modes may explain the peak values observed at 999 and 761 cm-1 [34]. Hmim's ring out-of-plane bending vibration was the cause of the 698 cm-1 band. The peak representation at 3430 cm-1 and 1586 cm-1 demonstrates the C-OH bond. The FT-IR spectrum of ZIF-67 samples produced at different Hmim/Zn molar ratios is indicated in Figure 6 (S2). At 3135 cm-1 and 2926 cm-1, respectively, two minor peaks correspond to the C-H stretching modes of vibration of the imidazole ring as well as the methyl group found in the linker. The intensity of ring stretches as a whole peaks at 1306 cm-1, while the intensity of C=N stretch modes peaks at 1578 cm-1. The stretching and plane bending vibrational peaks of the imidazole ring are found at 500-1500 cm-1. The bending and stretching vibration of related to the peak values at 754-692 cm-1 [35]. The absorption peak at Co-N stretching at 492 cm-1 in ZIF- 67 confirms that Co is bonded to the N of imidazole.
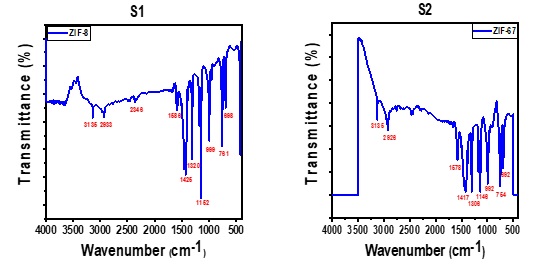
Figure 6. FTIR of ZIF-8 and ZIF-67
Raman shift of ZIF-8 and ZIF-67
The Raman spectra of ZIF-8 were observed to showcase a plethora of intriguing Raman spectra, each at distinct and fascinating peaks of 177, 681, 829, 945, 1019, 1130, 1178, 1302, 1368, 1445, 1496, and 1597 in Figure 7. The ethereal spectrums detected at 1130 and 1496 cm-1, in correspondence to the D and G bands, respectively, were unearthed in the Raman spectrum of ZIF-8 [35]. The ZIF-67 is observed to showcase a plethora of intriguing Raman spectra at 266, 394, 519, 614, 742, 941, 1038, 1116, 1178, 1387, 1407, 1445, 1760, and 1814. The spectrum detected D and G bands at 1116, and 1590 cm-1, respectively [36]. The slightly more pronounced peaks of ZIF-8 demonstrate its slight yet discernible dominance over ZIF-67 in the comparative Raman spectrum. It is important to note that the illustrious D and G bands are present in the spectra of both materials. The G peak occurs because of the stretching motion between sp2 carbon atoms in the plane. The D band is identified as a disordered band that arises from structural defects, edge effects, and broken symmetry in dangling sp2 carbon bonds.

Figure 7. Raman shift of ZIF-8 and ZIF-67
Electrochemical analysis of ZIF-8 and ZIF-67
In Figure 8 (W1 and W2), the electrochemical study of ZIF-8 and ZIF-67 was measured using three electrodes. To assess the electrochemical performance, tests were carried out during the CV measurements with 2 M KOH electrolyte at different scan speeds ranging from 10 to 50 mV/s. The electrodes showed impressive pseudo-capacitive performance with peaks in reduction/oxidation visible in the potential windows between -0.2 to 0.6 V. The specific capacitances of ZIF-8 and ZIF-67 were calculated via Equation (1) [37] using a CV plot.
![]()
The area of the material is denoted by A in Equation (1), while m represents an active mass on the nickel foam. The change in the potential window is indicated by dV and the scan rate is given by k. The presence of faradaic processes was demonstrated by the redox peaks in the CV plots. An increase in scan rate causes a reduction in the specific capacitance of both ZIF-8 and ZIF-67. The estimated specific capacitances for ZIF-8 and ZIF-67 are 625, 312, 208, 156, and 125 F/g and 932, 468, 312, 234, and 187 F/g at scan rates of 10 to 50 mV/s [38]. Applying potential causes the material to exhibit pseudo-capacitive properties. Faradaic reactions transfer charges quickly and in both directions. The ZIF-67 material displayed the highest specific capacitance according to the results. ZIF-67 material shows higher specific capacitance than ZIF-8. Specific capacitance quantifies a material's charge storage ability per unit mass or volume. ZIF-67's increased specific capacitance indicates that it has a greater energy storage capacity compared to ZIF-8. These differences may be attributed to variations in the structure, composition, or surface area of the two materials. Without more details, it's hard to pinpoint the exact causes for the disparity in specific capacitance between ZIF-67 and ZIF-8.
Figure 8 (W3 and W4) shows that both ZIF-8 and ZIF-67 were subjected to multiple tests during the EIS investigation. The CPE was utilized in evaluating resistance from the Nyquist plots, demonstrating a perfect match. The electrolyte resistances of ZIF-8 and ZIF-67 are 1.25 and 1.15 Ω, while their active electrode resistances are 0.03 and 0.02 Ω. The Nyquist plots showed low charge transfer resistance, indicating great capacitive performance. The slope of the Nyquist plot at low frequencies is referred to as Warburg resistance, which represents ion transport in the electrolyte. ZIF-8 and ZIF-67 materials proved to be excellent options for supercapacitor electrodes.
Galvanostatic Charge Discharge is useful for determining the specific capacitance, power, and energy densities of electrodes. At current densities of 2.0 A/g, this can be seen in Figure 8 (W5&W6). The GCD tests had the same electrolytic conditions as the CV measurements. The GCD tests show that specific capacitances of 276.91 F/g are estimated for both ZIF-8 and ZIF-67 [38]. The current density of 2.0 A/g led to an energy density of 7999.89 for ZIF-8 and ZIF-67. The IR drop, caused by the distance between electrodes, is the reason for the voltage change across the electrochemical interface.
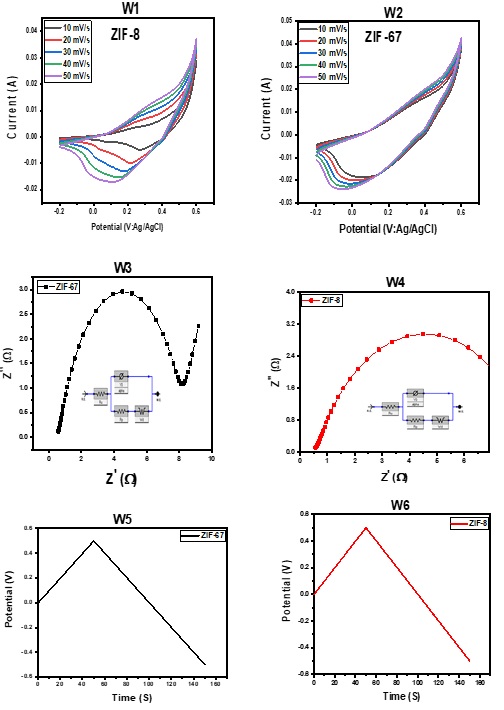
Figure 8. CV plots of ZIF-8 and ZIF-67
Conclusion
Synthesis of ZIF-8 and ZIF-67 for energy storage was successful using zinc nitrate hexahydrate, cobalt (II) nitrate hexahydrate, and 2-methyl imidazole, C4H6N2. The presence of faradaic processes was demonstrated by the redox peaks in the CV plots. An increase in scan rate results in a reduction of specific capacitance for both ZIF-8 and ZIF-67. The estimated specific capacitances for ZIF-8 and ZIF-67 are 625, 312, 208, 156, and 125 F/g and 932, 468, 312, 234, and 187 F/g at scan rates of 10 to 50 mV/s. The electrolyte resistances of ZIF-8 and ZIF-67 are 1.25 & 1.15 Ω, while their active electrode resistances are 0.03 and 0.02 Ω. The Nyquist plots showed low charge transfer resistance, indicating great capacitive performance. The GCD tests show that specific capacitances of 276.91 F/g are estimated for both ZIF-8 & ZIF-67. The current density of 2.0 A/g led to an energy density of 7999.89 for ZIF-8 and ZIF-67. XRD patterns, as the structure of ZIF-8, remain intact. The nanoparticles exhibit high crystallinity, as evidenced by the distinct peaks observed, particularly the sharp (001) peak at 7.4o. The crystals display a crystal lattice that is body-centered cubic. The integrity of ZIF-8's crystal structure remained intact, with no changes to its form. The ZIF-67 maintains its crystalline structure without any impurities. The high-pitched and defined peak at the 2θ angle of 7.6° confirmed the crystalline nature of synthesized ZIF-67. The (011) crystal plane was identified as the source of the intense peak at 2θ angle 7.6°. ZIF-67 has a bandgap value of 3.27 eV, while ZIF-8 has a bandgap value of 3.0 eV.
Disclosure of conflicting interest
The authors declare that they have no personal or financial conflicts that could have influenced the research described in this article.
Acknowledgments
Imosobomeh L. Ikhioya acknowledges the NCP-UNESCO-TWAS POSTDOCTORAL FELLOWSHIP FR no. 3240325060.
Orcid
Imosobomeh L. Ikhioya: https://orcid.org/0000-0002-5959-4427
Citation: S. Ibrar, E.O. Ojeguc, O.B. Odia, I.L. Ikhioya*, S. Afzal, M. Oneeb, I. Ahmad Assessing High-Performance Energy Storage of the Synthesized ZIF-8 and ZIF-67. J. Appl. Organomet. Chem., 2023, 3(4), 294-307.


