Document Type : Original Article
Authors
- Khaled Muftah Elsherif 1
- Mohamed Suliman Sasi 2
- Abdulfattah Mohamed Alkherraz 3
- Mariam Salem Elayeb 3
1 Libyan Authority for Scientific Research, Tripoli, Libya
2 Misurata University, Faculty of Education, Chemistry Department, Misurata, Libya
3 Misurata University, Faculty of Science, Chemistry Department, Misurata, Libya
Abstract
Alkaline hydrolysis of phosphate diester compounds with a common leaving group (LG) and varying non-leaving groups (NLG) was investigated. The nitrophenyl group was employed as a leaving group due to its favorable leaving group properties, significant absorbance for reaction monitoring, and selective cleavage of the phosphorus-oxygen bond, which is relevant for DNA and RNA degradation. Reactions were conducted under pseudo first-order conditions in NaOH solutions 70 °C. In addition, the alkaline hydrolysis was studied using a 1M NaOH solution at different temperatures (50–80 °C), pH (12-14) levels, and viscosities (with 0% - 40% glycerol). Reaction progress was continuously monitored by measuring the absorbance increase of the 4-nitrophenolate ion at λ = 400 nm. The rate constant (kobs) was determined by fitting the data to a first-order equation. The results demonstrate kobs increased with higher NaOH concentration, temperature, pH, and solution viscosity, except for NppNPP. Investigation of the effect of the non-leaving group's pKa revealed slight sensitivity of the hydrolysis reaction with βNLG = -0.12. Thermodynamic parameters were determined and the values were as follows: ∆H = 48.8 kJ.mol-1, ∆S = -171.6 J.mol-1.K-1, ∆G = 99.9 kJ.mol-1 for BpNPP; ∆H = 64.9 kJ.mol-1, ∆S = -127.1 J.mol-1.K-1, ∆G = 102.8 kJ.mol-1 for PypNPP; ∆H = 86.3 kJ.mol-1, ∆S = -76.7 J.mol-1.K-1, ∆G = 109.2 kJ.mol-1 for MpNPP, and ∆H = 63.7 kJ.mol-1, ∆S = -152 J.mol-1.K-1, and ∆G = 109 k kJ.mol-1 for NppNPP. All four hydrolysis processes have positive ∆G values, which means they are non-spontaneous and require external energy to proceed.
Graphical Abstract
Keywords
Main Subjects
Introduction
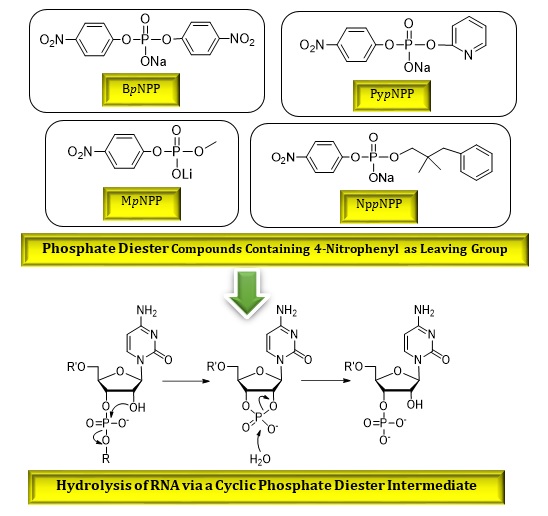
Phosphate esters are a significant type of biological functional groups [1]. They are involved in many vital processes in living systems, such as energy production, signaling, biosynthesis, and protein function regulation [1,2]. Phosphate diesters are the linkages that connect nucleic acids [3-5] and are the target of enzymes such as DNA polymerases. These enzymes break the phosphate diester bonds in DNA. These bonds are very stable in water and have a low rate of hydrolysis [6,7]. It is not possible to measure the hydrolysis reaction at the phosphorus site of natural compounds directly because phosphate esters in DNA and RNA have a very low reactivity [8]. Therefore, models that mimic biological phosphate diesters are used to study their intrinsic reactivity. Model studies have shown that the 2′–OH group in RNA makes the phosphate diester cleavage faster by about 109 times [9,10]. For RNA, the 2′–OH group attacks the phosphate diester intramolecularly (Figure 1) and forms a cyclic phosphate diester intermediate. This intermediate has a higher hydrolysis rate than acyclic diesters, which makes RNA degradation easier than DNA cleavage [11].

Figure 1. The hydrolysis of RNA via a cyclic phosphate diester intermediate with concomitant release of alcohol (-OR) [11]
Phosphate esters are essential for the stability of genetic information against hydrolysis [12]. DNA hydrolysis is very slow without specific enzymes and takes millions of years under mild conditions. Phosphate esters are formed when phosphoric acid reacts with alcohols and esterifies one or more positions of the phosphoric acid molecule. This produces phosphate monoesters, diesters, or triesters, depending on the number of esterified positions [13]. Phosphate monoesters are more reactive than diesters and triesters [14]. Phosphate diesters are the most stable phosphate esters in neutral conditions and have a low hydrolysis rate [15]. Phosphate ester hydrolysis can follow three different mechanisms (dissociative, associative, or concerted), but they all produce the same products when they react with a nucleophile [16-20]. Phosphate diesters with two alkyl groups have a high resistance to breaking down in neutral conditions [15]. This makes it hard to study how they react with water and measure their reaction rates [21]. This is mainly because they have a negative charge that is shared by two oxygen atoms that are the same. This charge makes the phosphorus atom less likely to attract other atoms than triesters [22]. Likewise, this charge acts like a shield that blocks other atoms from attacking and slows down the hydrolysis of phosphodiester bonds [23]. To see how stable the diester bond is in DNA, researchers look at how fast hydroxide ions attack dialkyl phosphate diesters [8]. The reactivity of phosphate diesters in water depends a lot on things like how basic the group that leaves is the type of attack by the other atom, and the formation of rings with five atoms [22]. Moreover, the group that does not leave can affect how active phosphate diesters are [24]. The hydrolysis rate of alkyl phosphate diesters was first found by studying dimethyl phosphate. Both breaking the P-O and C-O bonds give the same products, so they can only be told apart by using experiments with different isotopes. Parvinzadeh Gashti et al. [25] discovered that dimethyl phosphate is very resistant to alkaline hydrolysis in their investigation. They observed hydroxide ion attack rate constants of 6 x 10-6 M-1.s-1 at 125 °C and 2.2 x 10-6 M-1.s-1 at 115 °C. Extrapolating these data yielded a second-order rate constant of 3 x 10-11 M-1.s-1 for the hydroxide attack at 25°C. The particular location of cleavage, however, was not explored in that study. Following examinations on the location of cleavage [26], it was discovered that alkaline hydrolysis mostly resulted in 10% P-O cleavage, decreasing the prior estimate by a factor of 10. As a result, the estimated rate constant for phosphorous attack at 25 °C was changed to 6.8 x 10-12 M-1.s-1. Isotopic labelling experiments verified the breakage via P-O bonds [27]. Assuming that base catalysis is the primary reaction at pH 7, the predicted rate of hydrolysis for dialkyl diesters at neutral pH and 25 °C is 10-22 s-1, equivalent to a half-life of 1014 years. Williams and Wyman [8] used bis[2,2-dimethyl-3-benzoyl propan-1-ol] phosphate diester as their experimental paradigm to explore the base hydrolysis of phosphate diesters while avoiding hydrolysis via C-O cleavage. In this molecule, the neopentyl effect is used to sterically prevent assault at the carbon site while enabling attack at the phosphorus site. The hydrolysis of this molecule in 1 M KOH across a temperature range of 160-260 °C yielded an estimated second-order rate constant for base hydrolysis of 10-15 M-1.s-1 at 25 °C. This is a three-order-of-magnitude slower rate than prior estimates [8,28]. Schroeder et al. (2006) [4] also investigated the hydrolysis of dineopentyl phosphate using hydroxide ions. They extrapolated a half-life of around 15 million years at 25 °C, equating to a rate constant (kobs) of 1.4 x 10-15 s-1. Hosseinzadeh et al. [3] made an important contribution to understanding the reactivity of diaryl phosphate diesters in 1970. Their research is largely considered as the most important source of knowledge on the hydrolysis of diaryl phosphate diesters. They created a Brønsted plot at 100 °C using an activated succession of diaryl phosphate diesters spanning a pH range of 4 to 10. The diesters' pKa values varied from 4.07 to 8.35. Notably, they discovered khyd = 3.78 x 10-6 min-1 for the spontaneous hydrolysis of bis-4-nitrophenyl phosphate at 100 °C and I=1. This implies a half-life of more than 4 months at pH 3-4 and 100°C. Furthermore, they discovered that the basicity of the leaving group influences hydrolysis reactivity, as evidenced by a βLG value of -0.97 [3]. The rate constant for the non-enzymatic reaction between methyl-4-nitrophenyl phosphate and hydroxide ions at 42 °C was found by Zalatan and Herschlag in 2006 [29]. Kirby et al. (2013) [24] examined the hydrolysis of di-2-pyridyl Phosphate anion in CHES Buffer in 2013. They discovered that the rate constant for the compound's hydrolysis was 3.12 x 10-10 s-1 at pH 9.4, 25 °C, I=1, and 2.6 x 10-6 s-1 at pH 9.7, 25°C, I=1. They identified a number of possible mechanisms for this hydrolysis [21]. At 42 °C, hydroxide ions catalyzed the hydrolysis of a series of methyl aryl phosphate diesters, according to Zalatan and Herschlag's [29], new results on enzymatic catalysis involving alkaline phosphatase (AP) at 25 °C. At 42 °C, the hydroxide catalysis produced a βLG value of -0.94±0.05 and an ionic strength of 1 M. The hydrolysis of a range of aryl fluorophosphates was also investigated. According to the data, the hydrolysis process for this series of aryl fluorophosphates follows first-order kinetics in terms of hydroxide concentration (0.1 - 1.0 M NaOH). As the pKa of the parent phenol fell, the total rate of hydrolysis rose. The hydrolysis rate of 4-nitrophenyl fluorophosphates was determined to be 4.2 x 10-5 M-1.s-1 [30]. Using varying percentages of glycerol, Sasi and Alasefer (2015) [31] studied the influence of medium density on the hydrolysis of bis-(p-nitrophenyl) phosphate diesters. At 20 °C, they discovered that raising the glycerol percentage (from 0.0% to 25% in 1 M NaOH) boosted the reaction rate. They also discovered that the rate increased with increasing temperature. ∆H‡ = 39.02 kJ/mol and ∆S‡ = -149.9 J/K.mol were derived as thermodynamic parameters [32]. The aim of this study was to investigate at the kinetic hydrolysis of phosphate diesters (Bis-p-nitrophenyl phosphate diester (BpNPP), Pyridyl-p-nitrophenyl phosphate diester (PypNPP), Methyl-p-nitrophenyl phosphate diester (MpNPP), and Neopentyl phenyl-p-nitrophenyl phosphate diester (NppNPP)) with 4-nitrophenyl as the leaving group in alkaline solutions. The study aimed to determine the effect of various factors on the hydrolysis kinetics. These factors included adjusting the pH of the solution, increasing the solution viscosity using glycerol, assessing the effect of a non-leaving group, and testing the influence of temperature on the process. The study also intended to compute the thermodynamic parameters (ΔH, ΔS, and ΔG) connected with hydrolysis process.
Experimental
Chemical reagents
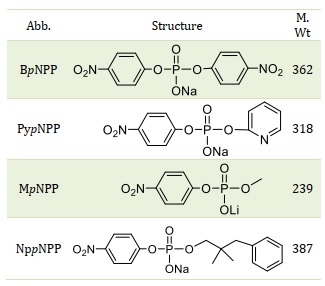
The compounds of interest, namely bis-p-nitrophenyl phosphate diester (BpNPP), pyridyl-p-nitrophenyl phosphate diester (PypNPP), methyl-p-nitrophenyl phosphate diester (MpNPP), and neopentyl phenyl-p-nitrophenyl phosphate diester (NppNPP), were synthesized following the methods outlined in the literature [31,32]. Sodium hydroxide (NaOH) of the highest available purity was obtained from Sigma Chemical Co., while sodium chloride (NaCl) and glycerol (99.5%) were purchased from Aldrich. Throughout the course of this study, deionized water was utilized Table 1.
Table 1. The structure of the compounds used in this study
Instruments
The UV-Vis spectrophotometer used in this study is JENWAY 6305, which has a wavelength range of 198 to 1000 nm and a bandwidth of 4 nm. The pH of the samples was monitored by JENWAY 3505, a bench pH meter with a resolution of 0.01. A water bath from Cole-Parmer WB-200 was used to keep the temperature constant during the measurements.
Kinetic study
All kinetic reactions were conducted in glass tubes, which were then immersed in a water bath. The temperature of the solutions in the water bath was continuously monitored and controlled within a narrow range (± 0.1 °C) using a thermometer.
The kinetic of phosphate diesters hydrolysis in alkaline solutions
The reactions were conducted under pseudo-first-order conditions [33]. To initiate the reaction, 50 µl (10 mM) of a substrate stock solution was added to 9950 µl of NaOH solution with varying concentrations (0.1, 0.2, 0.4, 0.8, and 1 M) in tightly sealed glass tubes. The initial substrate concentration in the reaction was 0.05 mM. The progress of the reactions was monitored by measuring the increase in absorbance of the 4-nitrophenolate ion at λ = 400 nm, using a spectrophotometer, at a temperature of 70 °C and an ionic strength of 1 M. For fast reactions, the reaction progress was followed for at least five half-lives. The observed first-order rate constants (kobs) were determined by fitting the absorbance vs. time curve to a nonlinear least-squares analysis, using the first-order equation (1) [34]:
![]()
For slow reactions, where the reaction progress was followed ≤ 10%, the rate constants (kobs) were obtained by dividing the slope of the straight line, obtained by plotting the absorbance of 4-nitrophenolate against time, by the absorbance of the total reaction (the initial rate method). The second-order rate constants were determined using linear regression analysis, by plotting the observed first-order rate constants against the concentration of the NaOH solutions [33,34].
Investigating the impact of non-leaving groups on hydrolysis reactions
In order to assess the influence of non-leaving groups, the observed rate constants (kobs) were plotted against the pKa values of the non-leaving groups. This analysis yielded the βNLG parameter, which provides insights into the impact of the non-leaving group on the hydrolysis of phosphate diesters.
The effect of pH on the hydrolysis of phosphate diesters
Under pseudo-first-order conditions, the reactions were initiated by adding 50 µl (10 mM) of a substrate stock solution to 9950 µl of NaOH solutions with concentrations of 0.01 M, 0.1 M, and 1 M, corresponding to pH values of 12, 13, and 14, respectively. The progress of the reactions was monitored as described earlier. The effects of pH were determined by plotting the natural logarithm of the observed rate constants (ln kobs) against the pH values.
Exploring the influence of solution viscosity on the hydrolysis of phosphate diesters
The experiments were conducted using 1M NaOH solutions with varying concentrations of glycerol (0%, 10%, 20%, and 40%) in a glycerol-water mixture. This allowed for the modification of the viscosity and density of the reaction medium by incorporating pure glycerol. The density (d) and viscosity (η) of the solutions were measured using a Pycnometer and Oswald's viscometer, respectively. The density and viscosity values were then calculated using the following formulas (2, 3) [33]:

Where, d denotes density of solution (g/mL), W is weight of solution (g), Vis volume of solution (mL), η1 indicates viscosity of solution (Pa.s), η2 is viscosity of water (at 60 °C = 0. 466 N.s.m-2), t1 is the time required to pass solution (s), t2: isthe time required to pass water (s), d1 is density of solution, and d2 indicates the density of water (at 60 °C = 0.98338 g/mL) The reactions were monitored at a temperature of 60 °C, with a substrate concentration of 0.05 mM and an ionic strength of 1 M. To investigate the impact of viscosity on the hydrolysis and rate constant, a nonlinear least-squares analysis was employed.
Investigating the influence of temperature on the hydrolysis of phosphate diesters
The progress of the reaction was monitored at temperatures of 50 °C, 60 °C, 70 °C, and 80 °C, with an ionic strength of 1 M. The observed rate constants (kobs) were determined. The activation parameters were then calculated using the linear Eyring equation (4, 5) [29,33]:

Where, k indicated observed rate constant (s-1), h is Blank’s constant (6.26 x 10-34 J.s-1), kB denotes Boltzmann’s constant (1.38 x 10-23 J.K-1), T isTemperature (K), and R is Gas constant (8.314 J.mol-1.K-1). An Eyring plot of ln () vs. () or (is linear. The plot can be used to obtain the standard entropy and enthalpy of activation from the intercept: and slope: – 3.
Results and Discussion
Kinetic analysis of phosphate diester hydrolysis in alkaline solutions
The hydrolysis of the targeted compounds was performed in various concentrations of NaOH (0.1 M, 0.2 M, 0.4 M, 0.8 M, and 1 M) under controlled conditions of 70 ± 1 °C and ionic strength of 1 M. The progress of the reaction was monitored by measuring the increase in absorbance of the 4-nitrophenolate ion at different time intervals. The absorbance-time relationship for the 4-nitrophenolate, acting as the leaving group, was utilized to determine the rate constant. The obtained rate constants (kobs) for the hydrolysis reaction of the studied compounds are summarized in Table 2. The results indicate that BpNPP exhibits the highest reactivity among the studied compounds, while NppNPP is the least reactive. Consequently, the reactivity ratio of the substrates is 1:1.5:6.5:27, respectively.
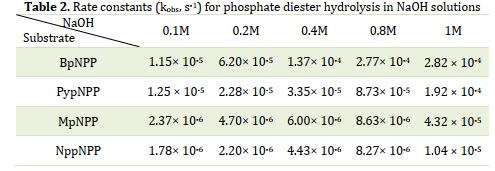
In a previous study [33], the hydroxide-catalyzed hydrolysis of three different diesters, namely bis-p-nitrophenyl phosphate, methyl-p-nitrophenyl phosphate, and neopentyl-p-nitrophenyl phosphate, exhibited distinct behaviors. The obtained rates at 95 °C were 3.25 × 10-3 s-1, 5.75 × 10-4 s-1, and 1.98 × 10-4 s-1, respectively. Comparing these values with the current study's results for the hydrolysis of three analogous phosphate diester monoanions at 70 °C, it was observed that the present values (2.82 × 10-4 s-1, 4.32 × 10-5 s-1, and 1.04 × 10-5 s-1, respectively) are at least 10 times lower than the previous values. The kobs values obtained in the present study are in good agreement with those reported in a previous study for BpNPP, which exhibited a kobs of 3.25 × 10-3 s-1 at 95 °C. In addition, the compound NppNPP displayed rates of kobs 4.88 × 10-7 s-1, 1.02 × 10-5 s-1, and 1.21 × 10-4 s-1 at 100 °C and pHs 12, 13, and 14, respectively [33].
Moreover, it was observed that the rate constant of the reaction increased with an increase in the concentration of NaOH, indicating that the hydrolysis reaction of the studied compounds follows a second-order reaction. The second-order rate constants (k2) were determined by analyzing the slope of the graph obtained by plotting the kobs values for each individual compound against the concentration of sodium hydroxide. The corresponding values are presented in Table 3. Notably, the k2 value obtained for the MpNPP compound in the current study closely matched the previous value reported for the same compound at 42 °C [29]. Similarly, the k2 value obtained for the BpNPP compound in the present study exhibited strong agreement with the previous value reported for Bis-2,4-DNPP at 39 °C [35].
Table 3. k2 for the hydrolysis of phosphate diesters
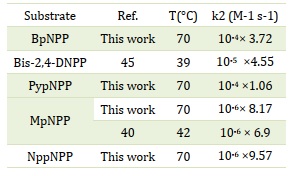
Furthermore, it is noteworthy that all the compounds investigated in this study exhibit significantly higher k2 values compared to dimethyl phosphate, as reported with rate constants of 6 × 10-6 M-1.s-1 at 125 °C and 2.2 × 10-6 M-1.s-1 at 115 °C for the attack of OH- [25].
Impact of non-Leaving group on hydrolysis reaction
One effective approach to investigate the influence of non-leaving groups (NLGs) on the hydrolysis of phosphate diesters and their rate constants is to examine a series of compounds that share a common good leaving group while possessing varying non-leaving groups. Interestingly, the rate constant of the reaction decreases as the pKa of the non-leaving group (βNLG) increases at different temperatures, as indicated by a value of -0.12 (Figure 2). This finding suggests that the impact of the non-leaving group on the hydrolysis reaction of the studied compound is relatively small or practically independent at high temperatures, which aligns with similar studies on aryl phosphate diesters mentioned previously [22, 36]. Despite the modest effects of non-leaving groups on the reaction rate, they still exert significant influence on the reactivity of phosphate hydrolysis [37]. 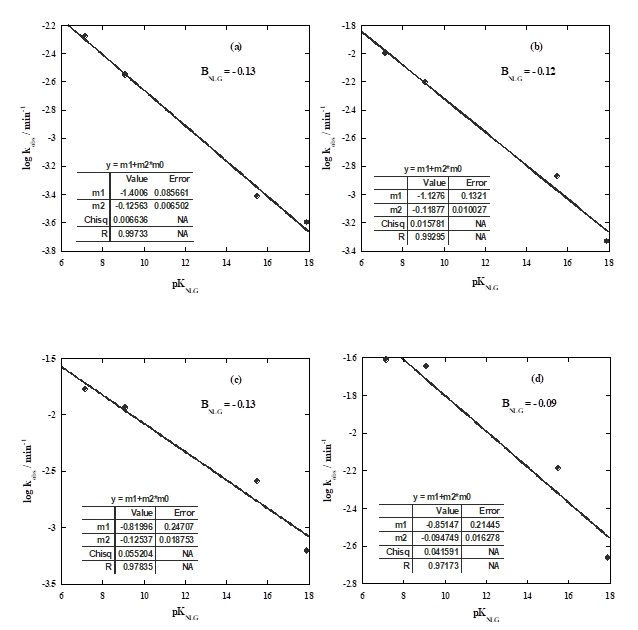
Figure 2. Correlation between log kobs and pKa for non-leaving groups at different temperatures: (a) 50 °C, (b) 60 °C, (c) 70 °C, and (d) 80 °C
Influence of pH on the hydrolysis of phosphate diesters
The alkaline hydrolysis of the targeted compounds was conducted at pH 12-14 and a temperature of 70 ± 1 °C. The progress of the reaction was monitored by measuring the increase in absorbance of the 4-nitrophenolate ion at different time intervals. The relationship between the absorbance of the 4-nitrophenolate, acting as the leaving group, and time was utilized to calculate the kobs values at each pH, which are presented in Table 4. The pH rate profile for the hydrolysis of the target compounds at 70 °C and an ionic strength of 1 M (Figure 3) demonstrates a distinct base catalysis effect, observed up to pH 12 (room temperature measurements). Moreover, the rate of the reaction increased as the pH was raised.
Table 4. Rate constants (kobs, s-1) for phosphate diester hydrolysis at various pH values and 70 ± 1 °C
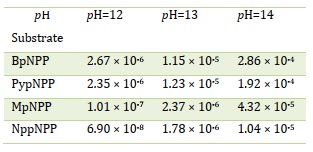

Figure 3. pH rate profile for phosphate diester hydrolysis at 70 °C and ionic strength 1M, (a) BpNPP, (b) PypNPP, (c) MpNPP, and (d) NppNPP
Impact of solution viscosity modification with glycerol addition on phosphate diester hydrolysis
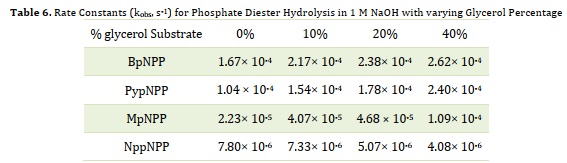
To investigate the impact of solution density changes, different percentages of pure glycerol were added to the NaOH solution. The density (d) and viscosity (η) values of the resulting solutions were calculated and presented in Table 5. The alkaline hydrolysis of the target compounds was then carried out at 60 ± 1 °C in 1 M NaOH solutions containing 0%, 10%, 20%, and 40% glycerol. The progress of the reaction was monitored by measuring the increase in absorbance of the 4-nitrophenolate ion at various time intervals. The relationship between the absorbance of the 4-nitrophenolate, serving as the leaving group, and time was used to calculate the kobs values, which are summarized in Table 6. The results indicate that the rate of hydrolysis reaction (kobs) generally increases with an increase in solution viscosity for all target compounds, except for NppNPP, where the rate constants decrease with increasing solution viscosity.
The kobs value obtained for the BpNPP compound at 60ºC with 10% glycerol (2.17 × 10-4 s-1) is twice as high as the previous value reported as 1 × 10-4 s-1 [31]. For the first three substrates, the kobs values increase in correspondence with the increasing pKa of the non-leaving group. However, the decrease in kobs for NppNPP may be attributed to the neopentyl effect and the size of the non-leaving group, which hinder the attack of the nucleophile at the phosphorus.
Table 5. Density and viscosity values of solutions
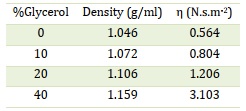
Table 6. Rate Constants (kobs, s-1) for Phosphate Diester Hydrolysis in 1 M NaOH with varying Glycerol Percentage
Influence of temperature on phosphate diester hydrolysis
To investigate the impact of temperature on the hydrolysis of the studied diester compounds and determine the activation parameters (∆H, ∆S, and ∆G), alkaline hydrolysis experiments were conducted in 1 M NaOH solution at various temperatures ranging from 50 °C to 80 ± 1 °C. The resulting rate constants (kobs) were obtained and presented in Table 7. The results revealed a significantly higher reaction rate at 80 °C compared to 70 °C, 60 °C, and 50 °C, indicating an endothermic reaction. By applying the Eyring equation and plotting ln(k·h/kB·T) against 1000/T, a linear least squares equation was fitted to the data. The activation parameters (∆H, ∆S, and ∆G) were then determined from the slope and intercept of the plot. The values of the activation parameters are summarized in Table 8. The results indicate that the Gibbs energy (∆G) has a positive value, suggesting that the reaction is non-spontaneous. The enthalpy (∆H) values range from 48.8 to 86.3 kJ.mol-1 [24], with the highest value observed for MpNPP and the lowest value for BpNPP. Interestingly, the ∆H value for BpNPP is similar to that of bis-2,4-DNPP, which is ΔH‡ 42.5 kJ.mol-1 [38]. In addition, all the ∆H values are positive, indicating an endothermic reaction. The entropy (∆S) values are negative, implying a non-spontaneous and bimolecular reaction under these specific conditions.
Table 7. Rate constants (kobs, s-1) for phosphate diester hydrolysis in 1 M NaOH at various temperatures
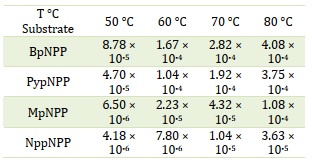
Table 8. Activation parameters at 25 °C for the hydrolysis of studied phosphate diesters anions

The variation in entropy values may be attributed to differences in the mechanisms among the compounds. The kobs values of the studied compounds were extrapolated at pH 14 and 37 ºC (the average human body temperature), resulting in values of 4.31 × 10-5 s-1 for BpNPP, 1.74 × 10-5 s-1 for PypNPP, 1.85 × 10-6 s-1 for MpNPP, and 1.39 × 10-6 s-1 for NppNPP.
The enthalpy value obtained in this study is lower compared to the previously reported value for bis-(2,4-dinitrophenyl) phosphate, which resulted in activation parameters of ΔH‡ 79.5 kJ.mol-1 and ΔS‡ -106.8 J.mol-1K-1 [3]. However, the entropy value is higher. The highly activated diester, methyl 2,4-dinitrophenyl phosphate [38], exhibits an activation entropy of ΔS‡ -131.8 J.mol-1.K-1 [28, 39-41].
Conclusion
Based on the findings of this study, it can be concluded that the rate constant (kobs) for alkaline hydrolysis of phosphate diester compounds generally increases with higher concentrations of NaOH solution, temperature, pH, and solution viscosity, except for NppNPP compound. In the case of NppNPP, the kobsdecrease with increasing solution viscosity, which can be attributed to the size of the non-leaving group. Furthermore, the influence of the pKa values of the non-leaving groups on the hydrolysis reaction was examined. The results indicate that the hydrolysis reaction is relatively unaffected by the pKa values of the non-leaving groups. The thermodynamic parameters (∆H, ∆S, and ∆G) were determined using the linear plot of the Eyring equation. The results demonstrate that all reactions are endothermic, non-spontaneous, and the highest entropy is observed for BpNPP.
Acknowledgements
We acknowledge the Chemistry Department, Faculty of Science, Misurata University, for their support during the research titled "The Effect of Non-Leaving Groups on the Alkaline Hydrolysis of Phosphate Diesters: A Kinetic and Thermodynamic Approach." We are grateful for their resources, guidance, and access to laboratory facilities, which were essential for conducting this study.
Orcid
Khaled Muftah Elsherif: https://orcid.org/0000-0002-3884-1804
Mohamed Suliman Sasi: https://orcid.org/0009-0008-2911-1595
Abdulfattah Mohamed Alkherraz: https://orcid.org/0009-0006-7182-5458
Mariam Salem Elayeb: https://orcid.org/0009-0005-4459-7405
Citation: K. Muftah Elsherif, M. Suliman Sasi, A. Mohamed Alkherraz, M. Salem Elayeb, Kinetic and Thermodynamic Aspects of Phosphate Diester Hydrolysis in Alkaline Solutions: The Role of Non-Leaving Groups. J. Appl. Organomet. Chem., 2024, 4(1), 1-13.
----------------------------------------------------------------------------------------------------------------------------------------------------
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company