Document Type : Original Article
Authors
- Rahadian Zainul 1, 2
- Fathatul Akrami 1, 2
- Sukardi Yusuf 3
- Alfadhlani Alfadhlani 4
- Hasanudin Hasanudin 5
- Doche Delson 6
- Riso Sari Mandeli 7
- Hasriwan Putra 8
- Jerri Mapanta 9
- Metla Sai Bhavani Sravan 10
- Azril Azril 11
- Abel Adekanmi Adeyi 12
1 Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Padang, Indonesia
2 Center for Advanced Material Processing, Artificial Intelligence, and Biophysic Informatics (CAMPBIOTICS), Universitas Negeri Padang, Indonesia
3 Operational Division, PT Putra Perkasa Abadi, Jakarta, Indonesia
4 Departement of Industrial Engineering, Faculty of Engineering, Universitas Andalas, Kampus Limau Manih, Padang, Sumatera Barat 25163, Indonesia
5 Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sriwijaya, Indralaya 30662, Indonesia
6 Department of Planning & Controlling Production, Unit Of Production Support, Section OF Aternative Fuel & Raw Material, PT Semen Padang, West Sumatera, Indonesia
7 Environmental Science, Postgraduate Programme, Universitas Negeri Padang, Padang, West Sumatera, Indonesia
8 Research Center for Transportation Technology, National Research And Innovation Agency (BRIN), Indonesia
9 Mining & Infrastructure Department, PT Bara Blasting Perkasa, Jakarta, Indonesia
10 Department of Biomedical Engineering, National Cheng Kung University, 1 University Road, Tainan City, 70101, Taiwan, ROC
11 Department of Biomedical Engineering, National Cheng Kung University, Tainan City, Taiwan
12 Department of Chemical and Petroleum Engineering, Afe Babalola University Ado-Ekiti (ABUAD), Ekiti State, Nigeria
Abstract
Manganese oxides have emerged as a focal point of extensive research due to their multifaceted roles in various scientific domains. This comprehensive review article delves into the diverse facets of manganese oxides, spanning from their crucial involvement in environmental geochemistry to their pivotal role in biogeochemical cycling. These minerals exhibit a broad spectrum of redox chemistry, from Mn(II) to Mn(IV) and Mn(VII), allowing them to partake in a variety of catalytic processes. The article explores the impact of pH on the stability and reactivity of different manganese species, illustrating the relevance of these compounds across the acidic to alkaline pH spectrum. Furthermore, it delves into their significance in advanced oxidation processes for water treatment, particularly the potent MnO4–/HSO3– system. The article also unveils the pivotal role of manganese oxides in anion-exchange membrane fuel cells and electrolyzers as alternatives to noble metal catalysts for oxygen reduction and evolution reactions. Lastly, this review underscores the importance of understanding the thermodynamic stability of various manganese species under different conditions. It serves as a valuable resource for researchers and scientists exploring the multifaceted world of manganese oxides and their diverse applications across various scientific disciplines.
Graphical Abstract
Keywords
- Manganese oxides
- Biogeochemical cycling
- Environmental geochemistry
- Redox chemistry
- Sustainable catalysis
Main Subjects
Introduction
General background
Manganese oxides, a class of minerals with diverse oxidation states and redox chemistry, play pivotal roles in a wide array of scientific disciplines [1]. Their prevalence in the Earth's crust, from the acidic to alkaline pH spectrum, underscores their importance in environmental geochemistry, biogeochemical cycling, and advanced oxidation processes for water treatment [2]. These compounds exhibit a unique ability to undergo redox reactions under various conditions, making them crucial in sustainable catalysis, particularly in anion-exchange membrane fuel cells and electrolyzer. Despite their versatile applications, there remains a need to further comprehend the thermodynamic stability and reactivity of different manganese species under varying environmental conditions. Bridging this knowledge gap is essential for harnessing the full potential of manganese oxides and optimizing their utilization in addressing contemporary challenges in energy and environmental science [3].
State of art
In recent years, research on manganese oxides has garnered significant attention due to their multifaceted applications and pivotal roles across various scientific disciplines. Manganese oxides, with their diverse oxidation states, have been a subject of extensive investigation, particularly in environmental geochemistry, biogeochemical cycling, and sustainable catalysis [4,5,6]. These minerals exhibit a remarkable ability to engage in redox chemistry, transitioning between Mn(II), Mn(III), Mn(IV), and Mn(VII), and consequently, they have found applications in advanced oxidation processes for water treatment. This is elegantly depicted in Figure 1, which shows a Frost diagram for manganese, illustrating the various oxidation states such as MnO4, HMnO4, H3MnO4, etc. along with their respective pH values. The diagram also includes MnO, Mn3+, MnO4 2+, MnO4, Mn2O3, Mn(OH), and MnO2, providing a comprehensive view of the redox properties of manganese. Consequently, manganese oxides have found applications in advanced oxidation processes for water treatment. Notably, the MnO4–/HSO3– system has emerged as a powerful tool for oxidizing organic contaminants at exceptionally high rates [7].
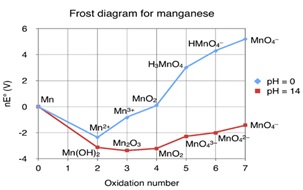
Figure 1. Frost diagram for manganese MnO4,HMnO4,4 H3MnO4, pH 14, Mn, MnO, Mn3+ MnO4 2+, MnO4, Mn2O3, Mn(OH), and MnO2 [13]
Moreover, manganese oxides have gained prominence in anion-exchange membrane fuel cells and electrolyzers as non-noble metal catalysts for oxygen reduction and evolution reactions, promising more sustainable energy conversion technologies. While these compounds offer versatile solutions, further exploration into the thermodynamic stability and reactivity of different manganese species under varying conditions is essential. Bridging this knowledge gap is critical to fully harness the potential of manganese oxides in addressing contemporary challenges in energy and environmental sciences [8,9,10,11].
Novelty
The primary novelty of this research lies in its comprehensive exploration of manganese oxides, shedding light on their versatile applications in environmental geochemistry, biogeochemical cycling, and sustainable catalysis. This study delves into the unique redox chemistry of manganese oxides, covering their diverse oxidation states, from Mn(II) to Mn(IV) and Mn(VII), and their implications for environmental processes [12].
In addition, the research uncovers the significance of manganese oxides in advanced oxidation technologies for water treatment, particularly the potent MnO4–/HSO3– system. Furthermore, this study highlights the crucial role of manganese oxides in anion-exchange membrane fuel cells and electrolyzers, offering sustainable alternatives to noble metal catalysts for oxygen reduction and evolution reactions. A key aspect of this research is outlined in Figure 2, which details the Mechanisms of manganese oxide electrocatalysts degradation during oxygen reduction and oxygen evolution. This section of the study is critical for understanding the durability and efficiency of manganese oxides in these applications, addressing the challenges related to their degradation during catalytic processes. The primary objective of this study is to gain a comprehensive understanding of the redox behavior and thermodynamic stability of manganese oxides under various environmental conditions, bridging the knowledge gap in their versatile applications [15].

Figure 2. Mechanisms of manganese oxide electrocatalysts degradation during oxygen reduction and oxygen [14]
Experimental Section
Research methods
The study will encompass a series of meticulously planned experimental steps. Initially, the initial phase involves the preparation of manganese oxide samples with various relevant oxidation states for this study, including Mn(II), Mn(III), Mn(IV), and Mn(VII). Sample preparation will be carried out through electrodeposition and specific treatments to achieve these different oxidation states. Subsequently, X-ray Absorption Spectroscopy (XAS) analysis will be conducted to monitor the structural changes and oxidation states of manganese oxide during redox reactions [16]. These experiments will be conducted under various environmental conditions, including a range of pH values from acidic to alkaline, to understand how these factors influence the reactivity and stability of manganese oxide [17]. Advanced analytical methods will be employed to identify various manganese species in solution during redox reactions. In addition, experiments will be conducted at different temperatures to comprehend the impact of temperature on the kinetics of redox reactions. An integral part of the methodology is illustrated in Figure 3, Experimental section diagram, which provides a comprehensive overview of the experimental setup and procedures. This diagram is essential for understanding the systematic approach taken in conducting the experiments, ensuring the reproducibility and reliability of the results. Thus, proper sample preparation and the analysis of various experimental parameters will be crucial steps in achieving the research objectives [18].
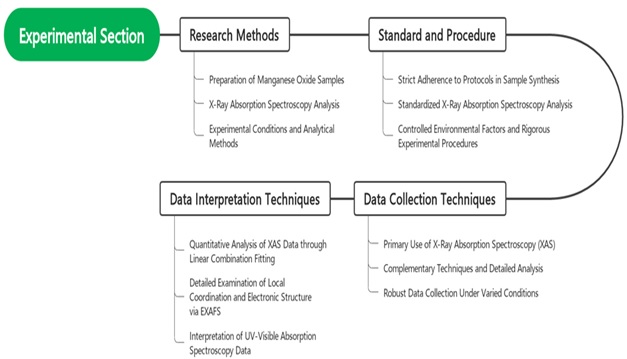
Figure 3. Experimental section diagram
Standard and procedure
The research will be conducted in accordance with established standards and procedures to ensure rigor and reproducibility. First and foremost, the synthesis of manganese oxide samples will adhere to strict protocols. This will involve the preparation of Mn(II), Mn(III), Mn(IV), and Mn(VII) samples through precise electrodeposition techniques and treatment processes, which have been widely accepted in the field [19]. The electrodeposition procedure will be conducted in controlled environments, maintaining a constant temperature and pH to ensure consistent results. The synthesis of manganese oxide samples will be executed with meticulous precision to eliminate any potential sources of contamination [20].
Furthermore, the analysis of manganese oxide samples during redox reactions will follow well-established methodologies. X-ray Absorption Spectroscopy (XAS) will be employed as a primary analytical technique. It is an established method for studying the oxidation states and structural changes of manganese compounds. XAS data will be collected using standard parameters and procedures, including the utilization of appropriate standards for calibration. Careful attention will be paid to XAS data collection under various experimental conditions, ensuring reproducibility and reliability [21]. To explore the influence of environmental factors, the research will adhere to standardized pH adjustments. The pH values will be meticulously controlled within a range from acidic to alkaline, conforming to widely recognized protocols in the field [22]. Temperature-dependent experiments will be executed with precision to evaluate the impact of temperature on the kinetics of redox reactions. The rigorous control of experimental parameters, adherence to established methods, and the consistent application of standards and procedures will underpin the robustness and reliability of the research findings. This systematic approach will ensure that the study contributes valid and insightful results to the field of manganese oxide research [23].
Data collection techniques
Data collection in this research will primarily rely on X-ray Absorption Spectroscopy (XAS) as the key analytical technique. XAS is a well-established method that provides invaluable insights into the oxidation states and structural transformations of manganese oxide samples during redox reactions. Data will be collected at the manganese L3,2-edges and the oxygen K-edge using inverse partial fluorescence yield (IPFY) and partial fluorescence yield (PFY), respectively [24]. To ensure the reliability and comparability of the data, standard reference materials and protocols will be employed for calibration. The experiments will be conducted under a range of controlled conditions, including variations in pH and temperature, to comprehensively assess the behavior of manganese oxide under different environmental scenarios [25]. The XAS data will be meticulously processed and analyzed, and spectral changes will be quantitatively interpreted to elucidate the redox behavior of manganese oxide species. In addition to XAS, ancillary techniques such as UV-Visible absorption spectroscopy and extended X-ray absorption fine structure (EXAFS) will be employed to complement the data collection. UV-Visible absorption spectroscopy will be used to monitor the formation and evolution of specific manganese oxide species during redox reactions. EXAFS will provide detailed information about the local coordination and electronic structure of manganese atoms in the oxide samples. The combination of these techniques will enable a comprehensive characterization of the manganese oxide system. An essential part of the analysis is exemplified in Figure 4, Mn K-edge XANES (Left) and EXAFS (Center) and Fourier transforms (Right) of oxides formed by two different amounts of MnxG complexes after incubation with Mn(II). This figure demonstrates the detailed spectral analysis conducted on manganese oxide samples incubated with different concentrations of MnxG complexes, thereby contributing to a deeper understanding of their structural and electronic properties. The data collected will be subjected to rigorous analysis, including linear combination fitting and Fourier transforms, to extract valuable information about the oxidation states, structural changes, and bonding interactions of manganese oxide species. The systematic application of these data collection techniques will ensure the acquisition of high-quality and robust data for the research [26].

Figure 4. Mn K-edge XANES (Left) and EXAFS (Center) and fourier transforms (Right) of oxides formed by two different amounts of MnxG complexes after incubation with Mn(II). (Left) XANES spectra of 50 µg·L-1 (solid bold line) and 5 µg·L-1 (dashed bold line) protein incubated in 100 µM Mn(II). XANES of MnCl2·4H2O and δ-MnO2 are provided as indicators for Mn(II) and Mn(IV) with maximum absorbance features at 6,553 and 6,562 eV, respectively. Mn K-edge EXAFS (Center) and fourier transforms (Right) of the two different concentrations of MnxG complex, 50 (a) and 5 (b) μg·L-1 [27].
Data interpretation techniques
Interpreting the data generated in this research necessitates a multifaceted approach. The X-ray Absorption Spectroscopy (XAS) data collected at the manganese L3,2-edges and oxygen K-edge will be meticulously analyzed using established procedures. Linear combination fitting will be employed to deconvolute the complex XAS spectra, quantitatively determining the relative contributions of different manganese oxidation states, such as Mn(II), Mn(III), and Mn(IV), in the samples [29].
This quantitative analysis will provide insights into the redox behavior of manganese oxide species during the redox reactions. Furthermore, the analysis of the extended X-ray absorption fine structure (EXAFS) data will offer a detailed examination of the local coordination and electronic structure of manganese atoms in the samples. Fourier transforms of EXAFS data will be used to reveal the characteristic features of manganese-oxygen bonding interactions, aiding in the comprehensive understanding of structural changes during redox processes. In this context, Figure 5, which presents Frost or oxidation state diagrams plot the relative free energy of a species versus oxidation state, will be particularly useful. This diagram serves as an invaluable tool for understanding the thermodynamics of the manganese redox system, enabling the correlation of the free energy of various manganese species with their respective oxidation states. Such diagrams assist in elucidating the stability and reactivity of different oxidation states, crucial for interpreting the redox chemistry of manganese oxides. The integration of these interpretation techniques will ensure a thorough understanding of the dynamic structural and electronic transformations occurring in manganese oxide species under various redox conditions. In addition, Figure 6 provides further insights into these processes. (Left) UV-Visible absorption spectra of purified oxidase in Hepes buffer, pH 7.5, in the presence of sodium pyrophosphate (PP) and O2, taken at the indicated time points after the addition of 0.1 mM MnCl2. (Right) Time courses followed by Mn(III)–PP and MnO2 species during Mn oxidation by purified oxidase and obtained from a linear least squares fit analysis of the time-resolved spectra, this figure illustrates the application of UV-Visible absorption spectroscopy in tracking the redox behavior of manganese species. The left part of the figure shows the changes in the absorption spectra of the oxidase-Mn complex over time, while the right part presents the quantitative analysis of the evolution of Mn(III)–PP and MnO2 species during the reaction. This data will be interpreted by tracking the changes in absorbance peaks, which correspond to the formation of specific manganese oxide species as the reactions progress [30]. The intensity, position, and shape of these peaks will provide valuable information regarding the evolution of manganese species. The integration of all these data sources will offer a holistic understanding of the redox behavior of manganese oxide and how it is influenced by pH and temperature variations. These interpretations will not only contribute to a deeper comprehension of the redox processes but will also aid in elucidating the roles and applications of manganese oxide in various scientific domains. The systematic application of these data interpretation techniques will ensure the extraction of meaningful and scientifically significant results from the research [31].

Figure 5. Frost or oxidation state diagrams plot the relative free energy of a species vs. oxidation state [28]
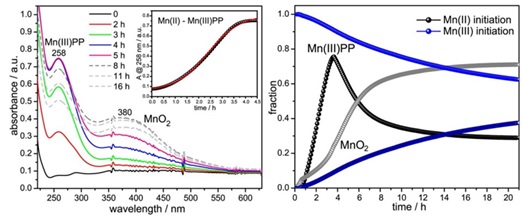
Figure 6. (Left) UV-Visible absorption spectra of purified oxidase in Hepes buffer, pH 7.5, in the presence of sodium pyrophosphate (PP) and O2, taken at the indicated time points after the addition of 0.1 mM MnCl2. (Right) Time courses followed by Mn(III)–PP and MnO2 species during Mn oxidation by purified oxidase and obtained from a linear least squares fit analysis of the time-resolved spectra [27]
Results and Discussion
Analysis
The study on manganese oxides, focusing on their diverse oxidation states and redox chemistry, holds significant implications across various scientific disciplines, from environmental geochemistry to sustainable catalysis. This investigation emphasizes the importance of understanding the thermodynamic stability and reactivity of manganese oxide species under different environmental conditions. The systematic exploration of manganese oxide's behavior across various oxidation states, including Mn(II), Mn(III), Mn(IV), and Mn(VII), presents a comprehensive approach to address critical questions in biogeochemical cycling, advanced oxidation processes, and energy conversion technologies [32].
Manganese oxides' unique redox chemistry enables them to function as efficient catalysts in diverse applications, such as the MnO4–/HSO3– system for organic contaminant oxidation and their role as non-noble metal catalysts in anion-exchange membrane fuel cells and electrolyzers. By studying the redox processes of manganese oxide under differing pH and temperature conditions, this research offers essential insights into their behavior in real-world scenarios. This information is invaluable for optimizing their applications, from environmental remediation to sustainable energy conversion [33]. The use of advanced analytical techniques, including X-ray Absorption Spectroscopy (XAS), UV-Visible absorption spectroscopy, and extended X-ray absorption fine structure (EXAFS), ensures the precision of data collection and interpretation. By adhering to established standards and procedures, this research guarantees the reliability of the acquired data. The findings will contribute to a deeper understanding of manganese oxide systems and their potential in addressing contemporary challenges in energy and environmental sciences. Figure 7 illustrates the effectiveness of the MnO4–/HSO3– system in oxidizing various organic contaminants, showcasing the practical applications of manganese oxides in environmental remediation. The data and insights obtained from this figure will be crucial in demonstrating the real-world efficacy of manganese oxide-based catalysis in pollutant degradation. Ultimately, this study will advance our knowledge of manganese oxide behavior and foster the development of more sustainable and effective solutions for environmental and energy-related problems [35].
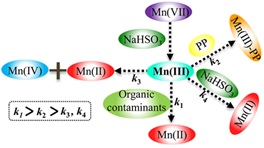
Figure 7. The permanganate/bisulfite (PM/BS) process oxidized phenol, ciprofloxacin, and methyl blue [34]
Interpretation research
The research outcomes provide a comprehensive understanding of manganese oxide's redox behavior and its potential applications across multiple scientific domains. The ability of manganese oxides to transition between various oxidation states, including Mn(II), Mn(III), Mn(IV), and Mn(VII), highlights their versatility and underscores their significance in environmental geochemistry, catalysis, and energy conversion technologies. The findings underscore the dynamic nature of these compounds, with reversible yet hysteretic Mn redox behavior observed during redox processes [36].
This understanding of manganese oxide's behavior is vital for optimizing their use in advanced oxidation processes for water treatment, where the MnO4–/HSO3– system proves to be an exceptionally potent tool for rapid organic contaminant oxidation [37]. Furthermore, manganese oxides emerge as promising catalysts in anion-exchange membrane fuel cells and electrolyzers, contributing to the development of sustainable energy conversion solutions [38]. Manganese oxides emerge as promising catalysts in anion-exchange membrane fuel cells and electrolyzers refers to recent developments in the field of renewable energy technology. Manganese oxides, or manganese oxides, have been discovered as promising catalysts in anion-exchange membrane fuel cell and electrolyzer applications. Anion exchange membrane fuel cells are technologies that allow for efficient and environmentally friendly energy production by converting fuels, such as hydrogen, into electricity. On the other hand, electrolyzers are devices used to split water into hydrogen and oxygen using electrical energy. The use of manganese oxide as a catalyst in this technology offers several advantages. Manganese oxide is cheaper and more abundant compared to conventional catalysts, such as platinum, which is often used in fuel cells. In addition, manganese oxide has good chemical and thermal stability, which is very important for long-term operation and high efficiency. This finding paves the way for the development of more economical and sustainable fuel cells and electrolyzers, which can play a key role in the global energy transition towards cleaner and more renewable sources.
The rigorous data collection techniques employed in this research, including X-ray Absorption Spectroscopy (XAS), UV-Visible absorption spectroscopy, and extended X-ray absorption fine structure (EXAFS), ensure the acquisition of high-quality and precise data [39]. The analysis of XAS spectra using linear combination fitting allows for the quantitative determination of manganese oxidation states, facilitating a deeper understanding of the involved redox processes. The investigations of UV-Visible absorption spectroscopy and EXAFS contribute to a holistic view of the structural changes and bonding interactions in manganese oxide species during redox reactions [40]. Furthermore, Figure 8 is an integral part of the research, aiding in the interpretation of data. This diagram maps the stability and solubility of manganese species at different pH and electrode potential levels. It is a crucial tool for understanding how manganese behaves in various environmental conditions, influencing its applications in catalysis and energy conversion. The Pourbaix diagram helps in predicting the forms of manganese under different conditions, essential for tailoring its applications effectively. The inclusion of this diagram in the research underscores the comprehensive approach taken in analyzing and understanding the redox behavior of manganese oxides. Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy plays a crucial role in providing a comprehensive understanding of structural changes and bonding interactions in various materials. For instance, consider a study on a newly developed catalyst used for environmental cleanup. EXAFS can be employed to investigate the structural changes that occur in the catalyst when exposed to different environmental conditions. By analyzing the absorption spectrum, scientists can determine how the atomic structure of the catalyst changes, identifying shifts in bond lengths and strengths. This information is invaluable in understanding how these structural changes influence the catalytic activity and stability. Furthermore, EXAFS can reveal how atoms in the catalyst interact with pollutant molecules at a molecular level, offering insights into the mechanisms of pollutant breakdown. Overall, the EXAFS ability to provide detailed information about atomic arrangements and bonding interactions in real-time and under varying conditions contributes significantly to a holistic understanding of material behavior and functionality.
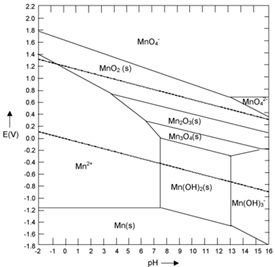
Figure 8 . Pourbaix diagram of manganese [28]
These comprehensive insights are crucial for harnessing the full potential of manganese oxides in addressing pressing challenges related to water treatment and sustainable energy conversion [41]. The research signifies the importance of temperature and pH in influencing the redox behavior of manganese oxide species. These parameters play a pivotal role in determining the kinetics and thermodynamic stability of the redox processes. Such knowledge is essential for the design and optimization of manganese oxide-based catalysts and technologies, with applications ranging from environmental remediation to green energy production [43]. The combined use of advanced analytical techniques and rigorous adherence to established standards and procedures ensures the reliability and robustness of the research outcomes. In addition, Figure 9 provides a key illustration of the core concepts in this study. This figure offers a clear and simplified representation of the complex redox reactions involving manganese. It shows the pathways through which manganese transitions between its different oxidation states in various chemical reactions. Understanding these pathways is vital for developing effective manganese oxide-based applications, as it guides researchers in manipulating the redox properties of manganese to achieve desired outcomes. This schematic representation aids in visualizing the mechanisms of manganese's redox activity, further enriching the understanding of its behavior under various environmental conditions. Overall, this research offers a critical foundation for harnessing the potential of manganese oxides in addressing contemporary environmental and energy-related challenges [44].
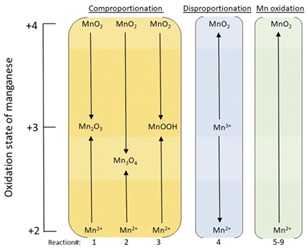
Figure 9. Schematic illustrating how the oxidation state of manganese changes for the comproportionation, disproportionation and Mn2+ oxidation reactions [42]
Comparative perspective
From various perspectives and viewpoints, the research on manganese oxides presents several notable comparisons. Firstly, when viewed within the realm of environmental geochemistry, this research enriches our understanding of manganese oxide's role in the redox cycling of elements in oxic and anoxic systems [45].
The findings align with existing knowledge, indicating that manganese and iron minerals undergo microbial oxidation and reduction under oxic and anoxic conditions, influencing the cycling of these elements [46]. Manganese and iron minerals undergo microbial oxidation, a fascinating process where microorganisms, particularly bacteria, play a crucial role in the geochemical cycling of these metals. This process is not just a simple chemical reaction; it is a complex interplay between biology and geology. Certain bacteria, known as chemolithotrophs, are capable of oxidizing manganese and iron as a means to generate energy for their survival. During this process, these bacteria oxidize the reduced forms of manganese and iron, Mn(II) and Fe(II), converting them into their oxidized forms, Mn(IV) and Fe(III), respectively. This oxidation results in the formation of minerals such as manganese oxides and ferric oxides. These minerals have significant environmental and industrial importance. For instance, in soil and aquatic systems, they influence nutrient cycling and can also act as sinks for various contaminants. In industrial contexts, understanding this microbial oxidation process is vital for developing strategies to manage corrosion, a common issue where iron is a primary structural material. This microbial influence on manganese and iron cycling illustrates a key aspect of how microorganisms can affect geological processes and material properties. In this regard, Figure 10 visually represents the intricate interactions and transformations of manganese and iron minerals under different environmental conditions. It highlights the transitions of these minerals between their various oxidation states, mediated by microbial activity, and the implications of these transformations in both oxic and anoxic environments. The inclusion of this figure in the research provides a more comprehensive understanding of the geochemical processes involved in the cycling of manganese and iron, shedding light on the broader environmental impact of these processes.
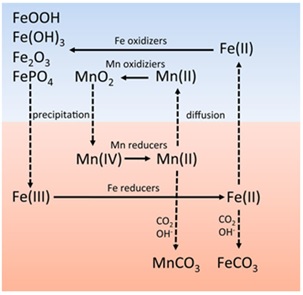
Figure 10. Manganese and iron minerals involved in redox cycling between oxic and anoxic systems [47]
The figure aids in visualizing the complex nature of manganese and iron redox cycling, thereby enhancing the overall understanding of their roles in environmental geochemistry and their interactions with biological systems. However, the research goes a step further by shedding light on the unique redox behavior and stability of manganese oxides under different pH and temperature conditions. This comparative aspect adds nuance to our understanding of how manganese oxides interact with their surrounding environment [48]. From the perspective of catalysis, the research positions manganese oxides as promising catalysts for advanced oxidation processes and energy conversion technologies. In comparison to other catalysts, manganese oxides, especially in the MnO4–/HSO3– system, exhibit extraordinarily high reactivity in the oxidation of organic contaminants. This superior reactivity, combined with their stable and reversible redox behavior, renders them as highly efficient and cost-effective catalysts [49]. When compared to conventional advanced oxidation processes for water treatment, the manganese oxide-based system shows a remarkable improvement in reaction rates. Moreover, in the context of energy conversion, manganese oxides emerge as competitive non-noble metal catalysts for oxygen reduction and evolution reactions in anion-exchange membrane fuel cells and electrolyzers. This comparison underscores the potential of manganese oxides to replace costly and less sustainable catalysts in these applications [50]. From a technical standpoint, the research integrates a wide array of analytical techniques, including X-ray Absorption Spectroscopy (XAS), UV-Visible absorption spectroscopy, and extended X-ray absorption fine structure (EXAFS), for data collection and interpretation. This multifaceted approach sets the research apart, allowing for a comprehensive investigation of manganese oxide behavior [51]. The data collection methods are meticulously controlled, adhering to established standards and procedures, ensuring the reliability and rigor of the results. This comparison highlights the systematic and rigorous nature of the research, which contributes to the robustness and scientific validity of the findings.
An important aspect of this research is illustrated in Figure 11 plays a critical role in explaining the processes involved in the reduction of manganese oxide, a process that is essential in understanding how manganese oxides can be manipulated and used in various industrial applications. The schematic provides a detailed visual representation of the steps and chemical reactions involved in the reduction process, showcasing the transformation of manganese oxide ore into more usable forms through interactions with elemental sulfur. The inclusion of this figure adds a deeper dimension to the research, offering clarity on the reduction mechanism of manganese oxide, which is fundamental in both environmental and industrial contexts. Overall, the study on manganese oxides draws insightful comparisons that expand our knowledge in environmental geochemistry, catalysis, and energy conversion, offering a promising path for future applications and studies in these fields [53].
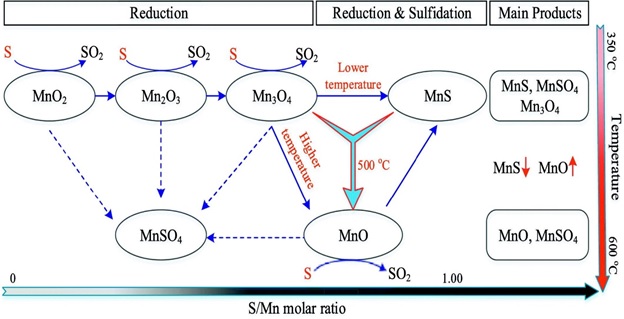
Figure 11. The mechanism on reducing manganese oxide ore with elemental sulfur [52]
Conclusion
The study on manganese oxides, with a focus on their diverse oxidation states and redox behavior, provides a comprehensive understanding of their potential applications in environmental geochemistry, catalysis, and energy conversion technologies. The findings underscore the importance of comprehending the stability and reactivity of manganese oxide species under varying pH and temperature conditions. Manganese oxides prove to be versatile and efficient catalysts, particularly in the MnO4–/HSO3– system for organic contaminant oxidation and as non-noble metal catalysts in anion-exchange membrane fuel cells and electrolyzers. The systematic application of advanced analytical techniques and adherence to established standards and procedures ensures the precision and rigor of the research. In summary, this study enhances our understanding of manganese oxides' redox chemistry and paves the way for their more sustainable and effective utilization in addressing contemporary environmental and energy-related challenges.
Acknowledgements
The authors extend their sincere gratitude to the Institute for Research and Community Services at Universitas Negeri Padang for their invaluable support and assistance throughout the course of this research.
Orcid
Rahadian Zainul: https://orcid.org/0000-0002-3740-3597
Fathatul Akrami: https://orcid.org/0009-0002-4074-4939
Sukardi Yusuf: https://orcid.org/0009-0006-8530-133X
Alfadhlani Alfadhlani: https://orcid.org/0009-0009-3919-1530
Hasanudin Hasanudin: https://orcid.org/0000-0003-2153-9163
Doche Delson: https://orcid.org/0009-0003-2025-9634
Riso Sari Mandeli: https://orcid.org/0009-0004-4170-9582
Hasriwan Putra: https://orcid.org/0000-0002-7718-7278
Jerri Mapanta: https://orcid.org/0000-0001-7646-4109
Metla Sai Bhavani Sravan: https://orcid.org/0000-0001-5705-2790
Azril Azril: https://orcid.org/0000-0001-8685-5517
Abel Adekanmi Adeyi: https://orcid.org/0000-0002-6428-0836
Citation: R. Zainul, F. Akrami, S. Yusuf, A. Alfadhlani, H. Hasanudin, D. Delson, R.S. Mandeli, H. Putra, J. Mapanta, M.S. Bhavani Sravan, A. Azril, A. Adekanmi Adeyi, Advances in Manganese Oxide Research: Environmental Geochemistry to Biogeochemical Cycling and Sustainable Catalysis. J. Appl. Organomet. Chem., 2024, 4(1), 14-29.
----------------------------------------------------------------------------------------------------------------------------------------------------
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company


