Document Type : Original Article
Authors
- Hana Bashir Shawish 1
- Khaled Muftah Elsherif 2
- Abdulfattah Mohamed Alkherraz 1
- Hanan Ibrahim Shuwat 1
- Eman Bashir Al-Melah 1
1 Department of Chemistry, Faculty of Science, Misurata University, Misurata, Libya
2 Libyan Authority for Scientific Research, Tripoli, Libya
Abstract
This study focuses on investigating the complexation process between N,N'-bis(salicylidene)ethylenediamine (Salen) and the metal complex [Cu(PDTC)2] in a dimethyl sulfoxide (DMSO) solvent. The kinetics and thermodynamics of the substitution reaction were examined. The [Cu(PDTC)2] complex and the Salen ligand were synthesized using a reported method, and their absorption spectra displayed characteristic peaks consistent with previous findings. The kinetics of the Cu(II) complex were studied under pseudo-first-order conditions in DMSO, with varying concentrations of Salen and a constant concentration of the [Cu(PDTC)2] complex. Reactions were carried out at temperatures of 25 °C, 30 °C, and 35 °C. By conducting temperature-dependent studies, the activation parameters (activation energy, activation entropy, and activation enthalpy) were determined. The substitution reaction was monitored through absorption spectra measurements, revealing a reduction in the absorption peak at 435 nm and the appearance of a new absorption peak at 360 nm. The rate constants obtained for the substitution reactions of salen at 25 °C fell within the range of 0.16x10-1 1/min to 5.66x10-1 1/min, which was higher compared to previous investigations due to the size of the substituted ligand. The reaction was found to follow the first-order kinetics with respect to [Cu(PDTC)2] and salen, indicating a second-order overall reaction. Increasing temperature resulted in higher values of kobs and k2. The calculated activation parameters revealed a positive activation entropy, implying a dissociative mechanism, and a positive activation enthalpy, indicating an endothermic nature of the substitution reaction.
Graphical Abstract
Keywords
Main Subjects
Introduction
Schiff bases represent a diverse array of compounds characterized by the presence of a double bond connecting carbon and nitrogen atoms. Their versatility stems from the numerous possibilities for combining various alkyl or aryl substituents. These compounds can be found in nature or synthesized in the laboratory. Over the years, Schiff bases have captivated chemists and biochemists due to their intriguing properties [1].
In the realm of coordination chemistry, Schiff bases serve as captivating polydentate ligands, exhibiting remarkable biological attributes. They have demonstrated antibacterial, antineoplastic, antimalarial, antioxidant, and antiviral activities [2-6]. Salen ligands, which are Schiff base ligands derived from salicylaldehyde and ethylenediamine, possess N2O2 donor atoms and have been observed to form square planar complexes. In the presence of additional ligands, square pyramidal and octahedral complexes can also be formed [7]. Diverse mono-, di-, or trinuclear Salen complexes have been synthesized using various transition metals, showcasing potential applications across a broad spectrum of fields, including catalysis, biochemistry, electrochemistry, sensors, molecular magnetism, and materials science [8-10].
The increasing interest in diverse chemical disciplines has propelled the exploration of mixed-ligand complexes, owing to their promising potential across various applications [11,12]. These complexes entail the coordination of more than one type of ligand to a singular central metal ion. The inclusion of multiple ligands within a complex engenders variations in its properties. In the synthesis of mixed-ligand complexes, adduct formation serves as a prevalent approach, wherein metal complexes interact with other Lewis base ligands, facilitating an elevation in the coordination number of metal ions while maintaining a consistent oxidation state [13]. Notably, square planar M(II) dithiocarbamates, denoted as [M(dtc)2], have exhibited structural transformations upon substitution of one dithiocarbamate anion with alternative ligands. This structural alteration entails the relocation of the dithiocarbamate species from its original position within the plane to an edge of the octahedral framework [14].
The substitution of a coordinated ligand with a free ligand in solution represents a notable phenomenon within the realm of coordination chemistry. Chelating ligands, characterized by their ability to form multiple bonds with the central metal ion, generally exhibit greater stability compared to monodentate ligands. Furthermore, cyclic ligands featuring the metal ion's donor atoms offer even higher levels of stability. Among the extensively studied chelating ligands, Salen-type ligands have garnered significant attention. These ligands form robust and inert complexes with a diverse range of transition metal ions [15,16]. While the structural aspects of mixed ligand complexes involving Salen Schiff bases have been extensively explored in solid-state investigations, limited information is available concerning their kinetics in solution [17].
The stability of a complex can be characterized by two key aspects: thermodynamic stability and kinetic stability. Thermodynamic stability pertains to the free energy change (∆G) associated with the formation of a complex, which relies on factors such as bond energy, stability constant, and redox potentials of the complex. On the other hand, kinetic stability refers to the rate of ligand substitution, influenced by parameters like activation energy, reaction mechanism, and intermediate complexes involved in the process. Complexes can exhibit either lability, indicating rapid ligand substitution, or inertness, denoting resistance to ligand exchange [18].
Several methodologies are employed to investigate the stability, kinetics, and thermodynamics of complex formation. Spectroscopic techniques, including UV-Vis, IR, and NMR spectroscopy, enable the examination of complex properties. Electrochemical techniques, such as cyclic voltammetry and potentiometry, provide insights into the behaviour of complexes. Calorimetric techniques, such as differential scanning calorimetry, offer valuable information regarding complex formation. Computational techniques, such as density functional theory, facilitate the exploration of complex structure, bonding, electronic properties, thermodynamic parameters, and reaction pathways. By employing these diverse methods, researchers can obtain comprehensive knowledge about complex characteristics [18-23].
This study presents a novel investigation into the complexation process between N,N'-bis(salicylidene)ethylenediamine (Salen) and the metal complex [Cu(PDTC)2] in a dimethyl sulfoxide (DMSO) solution. The kinetics analysis revealed higher rate constants for the substitution reactions of Salen with the [Cu(PDTC)2] complex, attributed to the size of the substituted ligand. Monitoring the reaction through absorption spectra measurements confirmed the formation of a new complex. In addition, temperature-dependent studies demonstrated an increase in rate constants with rising temperature. Moreover, the calculated activation parameters indicated a dissociative mechanism and an endothermic nature of the substitution reaction. These findings contribute valuable insights into the kinetics and thermodynamics of the complexation process, thereby enhancing our understanding of substitution reactions in coordination chemistry.
Drawing upon our previous investigations into Salen-type ligands, the present study aims to delve into the intricacies of the complexation process involving N,N′-bis(salicylidene)ethylenediamine (Salen) and the metal complex [Cu(PDTC)2] within a dimethyl sulfoxide (DMSO) solution. Our primary focus lies in scrutinizing both the kinetics and thermodynamics of the substitution reaction that takes place.
Experimental
Used chemicals
Analytical grade Copper(II) acetate (Cu(CH3COO)2.H2O), ethylenediamine, pyrrolidine dithiocarbamate, and salicylaldehyde obtained from Merck were employed to synthesize the ligands and complexes. The dimethyl sulfoxide (DMSO) utilized in the study was also of analytical grade and was used without any supplementary purification measures.
Preparation and reagents
The [Cu(PDTC)2] complex and the Salen ligand were synthesized using a previously reported method [24]. The absorption spectra of the [Cu(PDTC)2] complex displayed two distinct peaks at 435 and 679 nm, consistent with the published spectra [25].
The spectrophotometric kinetic measurements were conducted utilizing an Agilent Cary 60 UV-Vis Spectrophotometer. The absorbance within the visible spectrum, specifically within the charge transfer transition band, was monitored. The obtained curves were subsequently analyzed employing Excel and KaleidaGraph software.
Kinetic study
The kinetics of the Cu(II) complex were investigated utilizing pseudo-first-order conditions in dimethyl sulfoxide (DMSO), with an excess of Salen acting as the solvent. The concentration of the [Cu(PDTC)2] complex was maintained at a constant value of 1 x 10-4 M, while the concentration of Salen was systematically altered. The reactions were performed at three distinct temperatures: 25 °C, 30 °C, and 35 °C.
The data obtained from the experiment was analyzed using the linear least-square method. To determine the first-order rate constant (kobs), Equation (1) [26] was employed for the evaluation:
![]()
The absorbance at the beginning of the reaction (A0), at time t (At), and at the end of the reaction (A∞) were used in Equation (1) to determine the rate constant (k). The slope of the ln(At-A∞) versus time plot provided the rate constant.
Thermodynamic study
The kinetics of the reaction were investigated by conducting temperature-dependent studies in increments of 5 °C, ranging from 25 °C to 35 °C. Through this investigation, the kinetic activation parameters, such as the activation energy (Ea), activation entropy (ΔS‡), and activation enthalpy (ΔH‡), were determined. The rate constants obtained at various temperatures were fitted to both the Arrhenius Equation and the Eyring Equation [27] to evaluate these activation parameters:
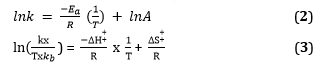
Where,
k is the rate constant, T is the temperature in Kelvin, kB is Boltzmann constant (1.380649×10-23 J/K), h is Planck constant (6.63×10-34 J/Hz), A is the pre-exponential factor or Arrhenius factor or frequency factor, ΔH‡ is the activation enthalpy, R is the gas constant (8.314 J/mol.K), and ΔS‡ is the activation entropy. A plot of ln k versus 1/T gives a straight line, whose gradient and intercept can be used to determine Ea and A.
Furthermore, through the construction of a plot of ln(k.h/T.kB) against 1/T, the slope and intercept of the resulting linear relationship can be ascertained. The slope corresponds to -ΔH‡/R, while the intercept is equivalent to ΔS‡/R. Hence, this linear representation of the Eyring Equation enables the determination of both the activation enthalpy and the activation entropy for the reaction. In addition, the Gibbs free energy of formation can be calculated using the subsequent equation (4) [20]:
![]()
Results and Discussion
Electronic absorption spectral study
Cachapa et al. [25] have documented the synthesis and characterization of copper(II) complexes containing bis(dithiocarbamato) ligands. The coordination environment around the copper(II) ions is described as a distorted square-pyramid. In the DMSO solvent, the copper(II) complex displays three notable absorption bands. The most prominent band at 273 nm is likely attributed to the ligand. The band observed at 438 nm corresponds to a (σ(S) → Cu(II)) ligand-to-metal charge transfer within a planar CuS4 chromophore. Furthermore, the band at 630-650 nm (shoulder) signifies a d-d transition.
In a separate study by Elsherif et al. [28], the complexation reaction between salen and Cu(II) was investigated in five different nonaqueous solvents. The free salen ligand exhibits an absorption maximum (λmax) at approximately 320 nm, which varies depending on the solvent. Upon chelation with Cu(II) ions, the absorption maxima (λmax) are shifted to 355-370 nm, depending on the specific employed solvent.
To monitor the substitution reaction between the [Cu(PDTC)2] complex and the Salen ligand, absorption spectra in the range of 300-600 nm were measured (Figures 1 (a, b, and c)). The substitution reaction leads to a reduction in the absorption peak at 435 nm, accompanied by the appearance of a new absorption peak at 360 nm [29]. Notably, Figure 1c indicates two isosbestic points, located at 330 nm and 400 nm, indicating the formation of a new complex resulting from the reaction between the copper complex and salen.
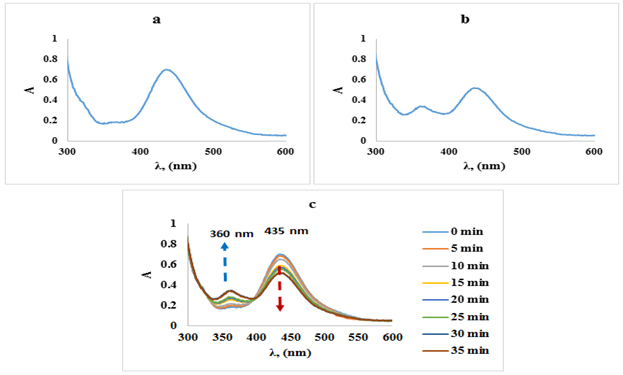
Figure 1. Absorption spectra of [Cu(PDTC)2]: (a) before substitution, (b) after substitution, and (c) after various times
Kinetic studies of the substitution reaction
The kinetics investigation of the substitution reaction between the [Cu(PDTC)2] complex and the salen ligand was conducted at temperatures of 25 °C, 30 °C, and 35 °C. According to the literature [30,31], it has been observed that copper complexes with PDTC and salen undergo a gradual weight loss as the temperature increases, indicating decomposition. In the initial stage, up to 100 °C, the mass loss ranged from 3% to 4.2%. [30,31]. The advancement of the reaction was monitored by observing the decline in absorbance at 435 nm, which corresponds to the maximum absorption of the initial complex. The reduction in absorption of the complex over time at 435 nm is depicted in Figure 2.
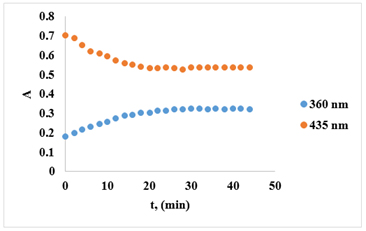
Figure 2. Complex absorption ([Cu(PDTC)2]) at 360 nm and 435 nm against time
The determination of rate constants was carried out at different temperatures and various concentrations of the salen ligand by plotting ln(At-A∞)/(A0- A∞) against time, as illustrated in Figures 3 (a, b, and c). In this representation, At represents the absorbance at time t, A∞ denotes the absorbance after the reaction has reached completion, and A0 signifies the initial absorbance. The slope of the linear plot yielded the estimated rate constants, designated as kobs, as presented in Table 1. In accordance with previous investigations [32-35], the rate constants for substitution reactions involving chloride ions and amine ligands typically fall within the range of 0.48x10-1 1/min to 9.00x10-1 1/min. In the present study, the obtained values at 25 °C ranged from 0.16x10-1 1/min to 5.66x10-1 1/min. The higher rate constants observed for salen can be attributed to the size of the substituted ligand, as the substitution of larger ligands tends to be favored.
Table 1. Pseudo first-order rate constants for the reaction of [Cu(PDTC)2] with different concentrations of salen in DMSO and various temperatures
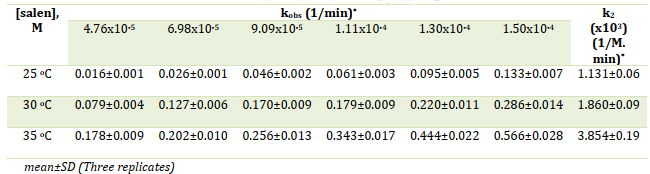

Figure 3. The pseudo first- order plots of ln [At-A∞/ Ao-A∞] vs. time for the complex at: (a) 25 oC, (b) 30 oC, and (c) 35 oC
The observed linearity of the plot provides strong evidence that the reaction follows first-order kinetics with respect to [Cu(PDTC)2]. In addition, a plot of ln(kobs) against ln[salen] (as shown in Figure 4) also exhibits a linear relationship, with a slope of 1.00, indicating that the reaction is first order with respect to salen. Consequently, the overall reaction is determined to be second order. This implies that the rate of the reaction is dependent on the concentrations of both the salen ligand and the substrate, which is consistent with findings reported in other studies [33-36]. Therefore, the rate equation (5) for the reaction is deduced to be as follow:
![]()
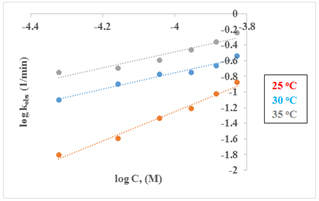
Figure 4. The plots of Logkobs vs. LogC in DMSO solvent at different temperatures
The information provided in Table 1 was utilized to construct a plot of kobs against [salen], enabling the determination of k2 values at each temperature. These calculated k2 values are presented in Table 1. The corresponding plots illustrating these results are depicted in Figure 5.
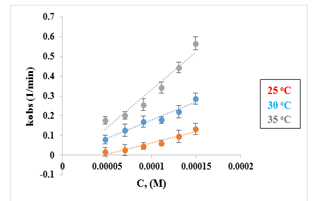
Figure 5. The plots of kobs vs. [salen] in DMSO and different temperatures
Temperature dependence rates of reaction
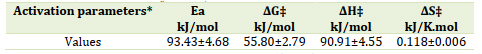
The investigation of the reaction was conducted within a temperature range of 25-35 °C (±0.1 °C). It was observed that both kobs and k2 increase with rising temperature, as summarized in Table 1. The activation parameters, namely Ea, ΔS‡, ΔH‡, and ΔG‡, were determined from the respective standard linear Arrhenius (lnk vs. 1/T) and Eyring plots (ln(k/T) vs. 1/T), exhibiting satisfactory correlation coefficients of 0.986 and 0.985, respectively. The resulting Arrhenius and Eyring plots are displayed in Figures 6 and 7, while the calculated activation parameters, including ΔG‡, ΔH‡, ΔS‡, and Ea, are provided in Table 2. Specifically, the values obtained were 55.80 kJ/mol, 90.91 kJ/mol, 0.118 kJ/mol.K, and 93.43 kJ/mol for ΔG‡, ΔH‡, ΔS‡, and Ea, respectively. The positive value of activation entropy (ΔS‡) suggests a dissociative mechanism for this substitution reaction [37]. Furthermore, the positive value of ΔH‡ indicates the endothermic nature of the substitution reaction. The findings presented in Table 2 indicate that the reaction exhibited characteristics of being nonspontaneous, endothermic, and causing a slight increase in disorder within the system. The positive value of the free Gibbs energy (55.80 kJ/mol) suggests that the reaction required external energy input to proceed and was not in a state of equilibrium. Moreover, the positive change in enthalpy (90.91 kJ/mol) indicates that the reaction absorbed heat from the surroundings, resulting in an increase in the system's internal energy. The change in entropy (0.118 kJ/mol) was small but positive, indicating a slight increase in the number of possible system configurations. However, this increase was not sufficient to counterbalance the unfavorable enthalpy change. The reaction's activation energy was determined to be high (93.43 kJ/mol), indicating the presence of a significant energy barrier that needed to be overcome for the reaction to take place [38-40].
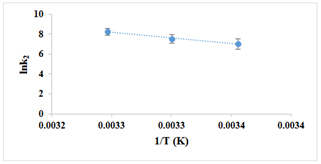
Figure 6. Arrhenius plot for the substitution reaction

Figure 7. Eyring plots for the substitution reaction
Table 2. Thermodynamic parameters of the substitution reaction
Conclusion
The present study focused on investigating the complexation process between N,N'-bis(salicylidene)ethylenediamine (Salen) and the metal complex [Cu(PDTC)2] in a dimethyl sulfoxide (DMSO) solution. Kinetics analysis revealed higher rate constants for the substitution reactions of Salen with the [Cu(PDTC)2] complex, primarily attributed to the size of the substituted ligand. Absorption spectra measurements were conducted to monitor the reaction, confirming the formation of a new complex. Temperature-dependent studies demonstrated an increase in rate constants as the temperature rose. The activation parameters, including ΔG‡, ΔH‡, ΔS‡, and Ea and the values were of 55.80 kJ/mol, 90.91 kJ/mol, 0.118 kJ/mol.K, and 93.43 kJ/mol for ΔG‡, ΔH‡, ΔS‡, and Ea, respectively. The positive value of the activation entropy (ΔS‡) suggests a dissociative mechanism for the substitution reaction. In addition, the positive value of ΔH‡ indicates the endothermic nature of the substitution reaction. It is worth noting that the rate constants obtained in this study at 25 °C ranged from 0.16x10-1 1/min to 5.66x10-1 1/min, which aligns with the typical range of rate constants for substitution reactions involving chloride ions and amine ligands. These findings provide valuable insights into the kinetics and thermodynamics of the complexation process, contributing to a better understanding of substitution reactions in coordination chemistry.
Acknowledgements
We acknowledge the Chemistry Department, Faculty of Science, Misurata University, for their support during the research titled " Investigation of Substitution Reaction Kinetics and Thermodynamics between Salen and [Cu(PDTC)2] Complex." We are grateful for their resources, guidance, and access to laboratory facilities, which were essential for conducting this study.
Orcid
Hana Bashir Shawish: https://orcid.org/0009-0006-4770-9721
Khaled Muftah Elsherif: https://orcid.org/0000-0002-3884-1804
Abdulfattah Mohamed Alkherraz: https://orcid.org/0009-0006-7182-5458
Hanan Ibrahim Shuwat: https://orcid.org/0009-0002-6855-3296
Eman Bashir Al-Melah: https://orcid.org/0009-0008-0724-8179
Citation: H.B. Shawish, K.M. Elsherif, A.M. Alkherraz, H.I. Shuwat, E.B. Al-Melah, Investigation of Substitution Reaction Kinetics and Thermodynamics between Salen and [Cu(PDTC)2] Complex. J. Appl. Organomet. Chem., 2024, 4(1), 51-61.
---------------------------------------------------------------------------------------------------------------------------------------------------
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company

![Investigation of Substitution Reaction Kinetics and Thermodynamics between Salen and [Cu(PDTC)2] Complex](data/jaoc/coversheet/991705843534.jpg)
